A recent study published in the journal eScience describes a novel solid-state electrolyte, Na4.92Y0.92Zr0.08Si4O12 (NYZS), which exhibits remarkable ionic conductivity and electrochemical stability at ambient temperature.
 Graphic abstract. Image Credit: TranSpread
Graphic abstract. Image Credit: TranSpread
The growing demand for renewable energy highlights the need for efficient and cost-effective energy storage technologies. Solid-state sodium batteries (SSSBs) provide significant economic and safety benefits, particularly in large-scale grid applications.
However, their broad acceptance is hampered by difficulties in producing high ionic conductivity in solid-state electrolytes, which are critical for efficient energy transfer and storage and have a major emphasis on advanced battery technology development.
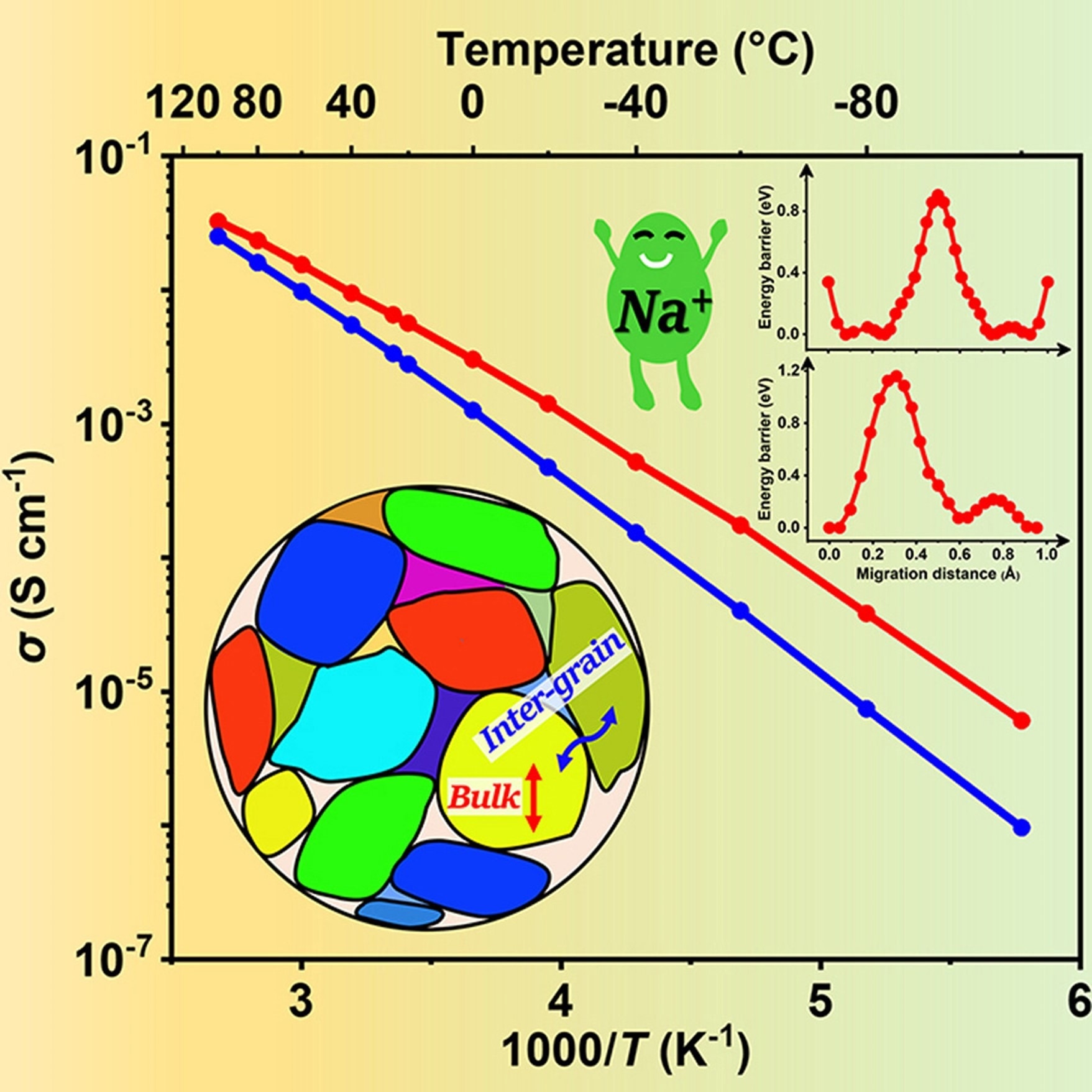
This novel material significantly improves the efficient conduction of ions at room temperature, which is critical for practical energy storage applications. The study team made this breakthrough by replacing a small amount of yttrium (Y) with zirconium (Zr) in the crystal structure of the existing material, resulting in an optimal arrangement that promotes the passage of sodium ions.
Ionic conductivity increased significantly as a result of this technique, up to 3.3 mS cm–1 for total conductivity and 6.5 mS cm–1 for bulk conductivity at room temperature. These values are among the highest that have ever been found for sodium superionic conductors.
In addition to its strong ionic conductivity, NYZS shows exceptional electrochemical stability and can resist voltages exceeding 10 volts versus Na+/Na. This ensures safer battery operations in a variety of scenarios.
The NYZS solid electrolyte represents a transformative step in the development of sodium-based energy-storage technologies. It not only supports superior conductivity and stability but is also compatible with scalable manufacturing processes, making it a highly promising material for future energy-storage solutions.
Dr Sylvio Indris, Study Corresponding Author and Senior Researcher, Karlsruhe Institute of Technology
This study marks a big step forward in the development of sodium-ion batteries for stationary energy storage. It can potentially improve the stability and efficiency of sodium-ion batteries, lowering dependency on expensive materials like lithium and cobalt, which are extensively utilized in existing battery technologies.
Study authors Aikai Yang, Hang Li, and Qiongqiong Lu are thankful to the China Scholarship Council (CSC, Grant Nos. 201906200023, 201906200016, and 201808080137, respectively) for financial assistance.
Yang, whose CSC grant application is linked with Nankai University (Tianjin, China), would like to convey his heartfelt thanks to Nankai University’s Key Laboratory of Advanced Energy Materials Chemistry (AEMC). Dr. Yoo Jung Sohn and Mr. Volker Bader assisted the authors with temperature-dependent XRD measurements and heat treatments.
The authors thank DESY (Hamburg, Germany), a member of the Helmholtz Association HGF, for providing the experimental facilities. Parts of this research were conducted at the PETRA III beamline P02.1.
Beamtime was assigned by an in-house team. X.S. gratefully welcomes financing from the European Union's Horizon 2020 research and innovation program under the Marie Sklodowska-Curie grant agreement (No. 101034329) and the Normandy Region's WINNINGNormandy Program. The authors accept responsibility for the contents of this publication.
Journal Reference:
Yang, A., et. al. (2024) Enhanced room-temperature Na+ ionic conductivity in Na4.92Y0.92Zr0.08Si4O12. eScience. doi:10.1016/j.esci.2023.100175.