Reviewed by Lexie CornerApr 30 2024
Prof. Tierui Zhang and Prof. Run Shi from the Technical Institute of Physics and Chemistry of the Chinese Academy of Sciences have successfully synthesized high-purity benzaldehyde compounds directly from the selective electrooxidation of benzyl alcohol, as described in a study published in Science Advances.
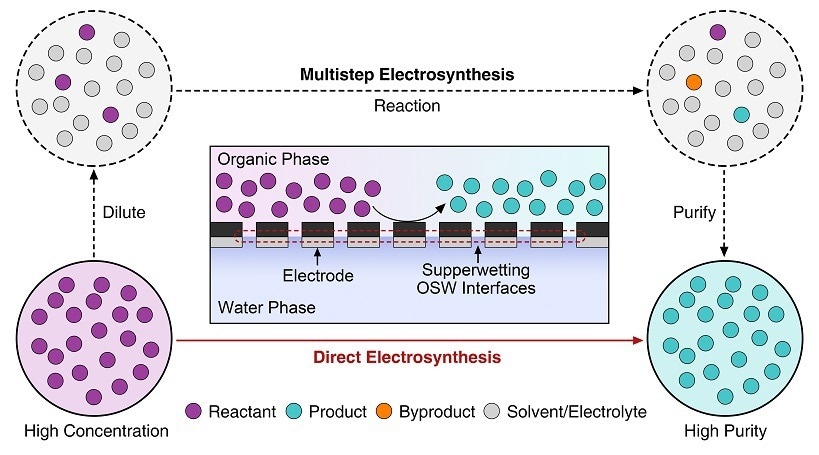 Electrosynthesis routes analysis. Image Credit: ZHANG’s group
Electrosynthesis routes analysis. Image Credit: ZHANG’s group
Compared to traditional H-type electrochemical cells, the organic-solid-water (OSW) three-phase reaction system has demonstrated unique benefits in lowering ohmic losses and streamlining the procedures involved in product separation and purification.
Water is a clean hydrogen and oxygen resource. Hence, electrocatalysis in an aqueous electrolyte has been acknowledged as a green technique for organic redox processes. However, due to the low solubility of many organics in the aqueous phase, most dilution-reaction-purification methods require several steps. As a result, there is still a barrier to the widespread use of electrocatalysis in the organic synthesis community.
For example, the electrooxidation of benzyl alcohol to benzaldehyde typically requires nonaqueous supporting electrolytes and solvents, which significantly increases the reaction overpotential, complicates the purification process, and causes overoxidation to acid during prolonged electrolysis.
Tierui Zhang, Professor, Technical Institute of Physics and Chemistry, Chinese Academy of Sciences
“The goal of our research is to develop a three-phase OSW electrocatalytic system for the efficient production of high-purity benzaldehyde. The new system features separate organic and water phases instead of an organic-in-water configuration in conventional H-type cells. It allows rapid supply of concentrated benzyl alcohol directly from the organic phase,” stated Prof. Run Shi.
Benzyl alcohol (9.3 mol/L) was employed in this study at concentrations more than 90 times greater than those used in other experiments. Without the need for any post-purification procedures, it produced high-purity benzaldehyde (91.7 %) in the organic phase and attained a 97 % Faradaic efficiency for benzaldehyde synthesis.
Electrodes with superwetting OSW interfaces can spontaneously separate organic and aqueous solutions. In this situation, the benzaldehyde product is recovered straight from the organic phase. This has two advantages: it prevents benzaldehyde overoxidation with an aqueous electrolyte and yields high-quality benzaldehyde when benzyl alcohol is entirely converted in the organic phase.
Electrosynthesis using superwetting OSW interfaces is, therefore, a suitable method for producing high-purity organic compounds. Even better, it employs clean energy and water resources, making the reaction more efficient and simpler.
This study was funded by the National Key Research and Development Program of China, the National Natural Science Foundation of China, and the CAS Project for Young Scientists in Basic Research.
Journal Reference:
Shi, R., et. al. (2024) Electrochemical oxidation of concentrated benzyl alcohol to high-purity benzaldehyde via superwetting organic-solid-water interfaces. Science Advances. doi:10.1126/sciadv.adn0947