Reviewed by Lexie CornerMay 17 2024
The use of greenhouse gasses is one of the most popular directions of the global trend toward decarbonization — reducing the carbon footprint from production and human activities. In the process of converting carbon dioxide into methane, the main catalysts used today are gold, platinum, and palladium, which are expensive and complex. A research group from Skoltech, Boreskov Institute of Catalysis (Siberian Branch of the Russian Academy of Sciences), and Tomsk Polytechnic University conducted an experiment and proved that a new photocatalyst based on WB5-x-WB2 tungsten boride and TiO2 titanium dioxide can compete with noble metals. It significantly increases the efficiency of chemical reactions and is much cheaper than the catalysts used today. The results are presented in a new study in the Applied Surface Science journal.
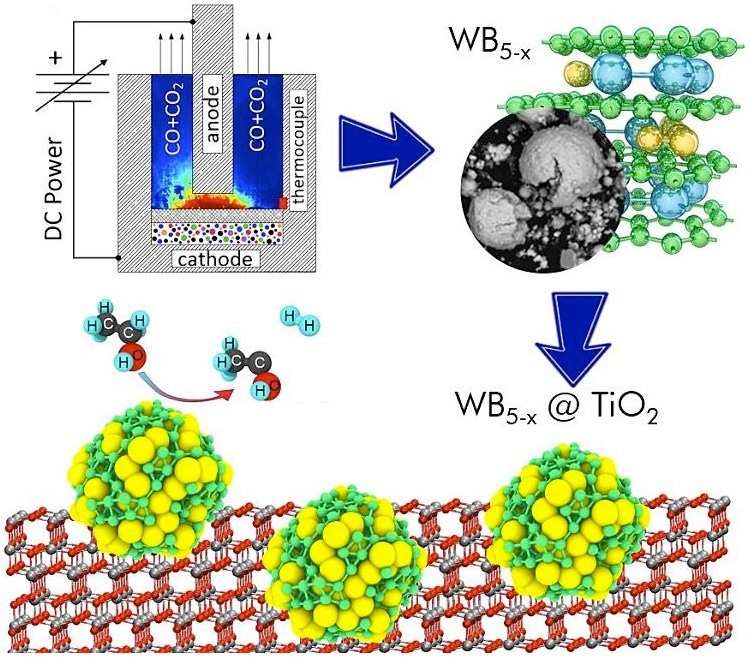 Graphical abstract of the study. Image Credit: Anna Kurenkova et al./Applied Surface Science
Graphical abstract of the study. Image Credit: Anna Kurenkova et al./Applied Surface Science
“We have identified properties that allowed us to assume that tungsten pentaboride is not only promising for oil production, but can also become a good catalyst. In the past, we only knew the crystal structure, stability information, and mechanical properties of the material. We have put in a lot of effort to predict the adsorption and catalytic properties of tungsten pentaboride through computer modeling and calculate the reaction barriers. Then we turned to our colleagues, who confirmed the results experimentally,” shared Aleksandra Radina, a study co-author and a PhD student at Skoltech’s Materials Science program.
Researchers from Tomsk Polytechnic University synthesized a powder of higher tungsten boride using a previously developed technology, while their colleagues from Boreskov Institute of Catalysis used the synthesized material as a cocatalyst for two reactions — converting carbon dioxide into methane and producing hydrogen from an aqueous solution of ethanol. According to the results, WB5-x-WB2 tungsten boride increased the efficiency of the first reaction by a factor of four and that of the second by a factor of 23. Structural analysis methods such as high-resolution transmission electron microscopy, X-ray diffraction, X-ray photoelectron spectroscopy, and others have confirmed the new WB5-x-WB2/TiO2 catalyst is responsible for increased reaction efficiency. Studies using these analytical techniques were performed at Boreskov Institute of Catalysis of the Siberian Branch of the Russian Academy of Sciences.
“The simulation data showed that the higher tungsten boride should work as an active catalyst material for producing hydrogen from ethanol, and the experimental results confirmed our predictions. As our material has not been previously considered as a catalyst, the question of screening chemical processes arises, where it could prove to be a more effective catalyst compared to traditional materials,” said Professor Alexander Kvashnin from Skoltech’s Energy Transition Center, the leader of the study.
As the authors point out, the new photocatalyst can be effective not only in the reactions considered above. Most importantly, the research opens up a new direction for applying materials based on borides and carbides of transition metals, including high-entropy ones. Currently, a team from three organizations is actively exploring the use of new materials in various catalytic processes with applications in photocatalysis, petrochemistry, and so on.
The work was supported by the Russian Ministry of Higher Education and Science under Project No. FSWW-2022-0018 and the Russian Academy of Sciences Grant No. 21-73-10235 titled “Obtaining valuable organic compounds by photocatalytic reduction of CO2: The structure of catalysts and the mechanism of their catalytic action.”