In a study recently published in the Chinese Journal of Catalysis, Professors Shaobin Wang from The University of Adelaide and Hui Zhang from Wuhan University explained the mechanisms of PMS activation by single-atom iron catalysts. They also identified the relationships between the geometric and electronic structures of single-atom Fe centers and the selective production of various reactive species/pathways.
 Single-atom catalysts revolutionize water purification: efficient and selective generation of reactive oxygen species for global water challenges. Researchers detail how downsizing active sites to the atomic scale in single-atom catalysts enhances the efficiency and selectivity of the generation of reactive oxygen species through peroxymonosulfate-based advanced oxidation. Their Review Article elucidates the underlying mechanism and identifies critical structure-activity/selectivity relationships, providing valuable insights into the rational design of catalysts for environmental remediation. Image Credit: Chinese Journal of Catalysis
Single-atom catalysts revolutionize water purification: efficient and selective generation of reactive oxygen species for global water challenges. Researchers detail how downsizing active sites to the atomic scale in single-atom catalysts enhances the efficiency and selectivity of the generation of reactive oxygen species through peroxymonosulfate-based advanced oxidation. Their Review Article elucidates the underlying mechanism and identifies critical structure-activity/selectivity relationships, providing valuable insights into the rational design of catalysts for environmental remediation. Image Credit: Chinese Journal of Catalysis
Excessive hazardous chemicals disposed of into the environment due to the centuries-long, fast increase in global industrialization significantly threaten aquatic ecology and human health.
Advanced oxidation processes based on peroxymonosulfate (PMS-AOPs) are attractive methods for treating these harmful contaminants. Aggressive Organic Pollutants (AOPs) are intended to oxidize or even mineralize by utilizing a variety of Reactive Oxygen Species (ROS).
With their homogeneous and well-defined active sites and optimal atom utilization, Single-Atom Catalysts (SACs) show considerable potential for efficient and targeted PMS activation. However, because of the numerous ROS production pathways and their intricate interactions, the structure-activity/selectivity correlations have not yet been fully elucidated.
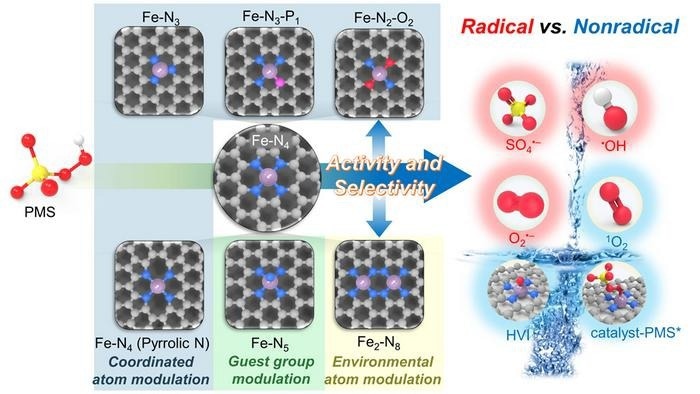
Three steps are typically involved in the activation of PMS by single-atom sites. The adsorption of HSO5- onto the single atom Fe core with different molecular alignments is the initial stage.
The second stage is the charge transfer between the adsorbed HSO5- and the active Fe center, which causes a shift in charge density and even the cleavage of the HSO5–O bond. To rebuild the active site, the third phase involves the detachment of the activated HSO5- through either a spontaneous release of reactive species (like free radicals and 1O2) or the breakdown of surface-bound species with additional substrates (like catalyst-PMS* and FeIV=O).
Diverse ROS are created even at a single atomic Fe site because PMS activation involves many stages, and ROS formation involves multiple chemical intermediates. Although the energetically ideal PMS adsorption structure may be effectively found theoretically, each reaction step must have a reasonable reaction-free energy for generating specific ROS.
As a result, controlling the PMS adsorption energy and the reaction-free energy of essential basic processes is necessary to control ROS selectivity. In this case, controlling the active site's electronic structure is useful for managing PMS activation activity and ROS selectivity to construct the connections.
Creating Fe–N2–O2 and Fe–N5 sites to improve 1O2 and FeIV =O creation, as well as a Fe–N3 site for catalyst-PMS complex generation, entails a moderate increase in the charge density of Fe sites. Radical production is favored by additional elevation of the charge density via Fe–N3–P1 and Fe–N4 (pyrrolic N) sites.
The Australian Research Council and the National Natural Science Foundation of China funded the research. The China Scholarship Council also gratefully acknowledged a one-year research grant at The University of Adelaide.
Journal Reference:
Cheng, C., et al. (2024) Single-atom iron catalysts for peroxymonosulfate-based advanced oxidation processes: Coordination structure versus reactive species. Chinese Journal of Catalysis. doi.org/10.1016/s1872-2067(23)64611-x.