Researchers from various universities, including North Carolina State University, developed a new class of materials known as "glassy gels" containing more than 50% liquid. These gels are incredibly hard and difficult to break. They are easy to make and have the potential for many uses. The research was published in the journal Nature.
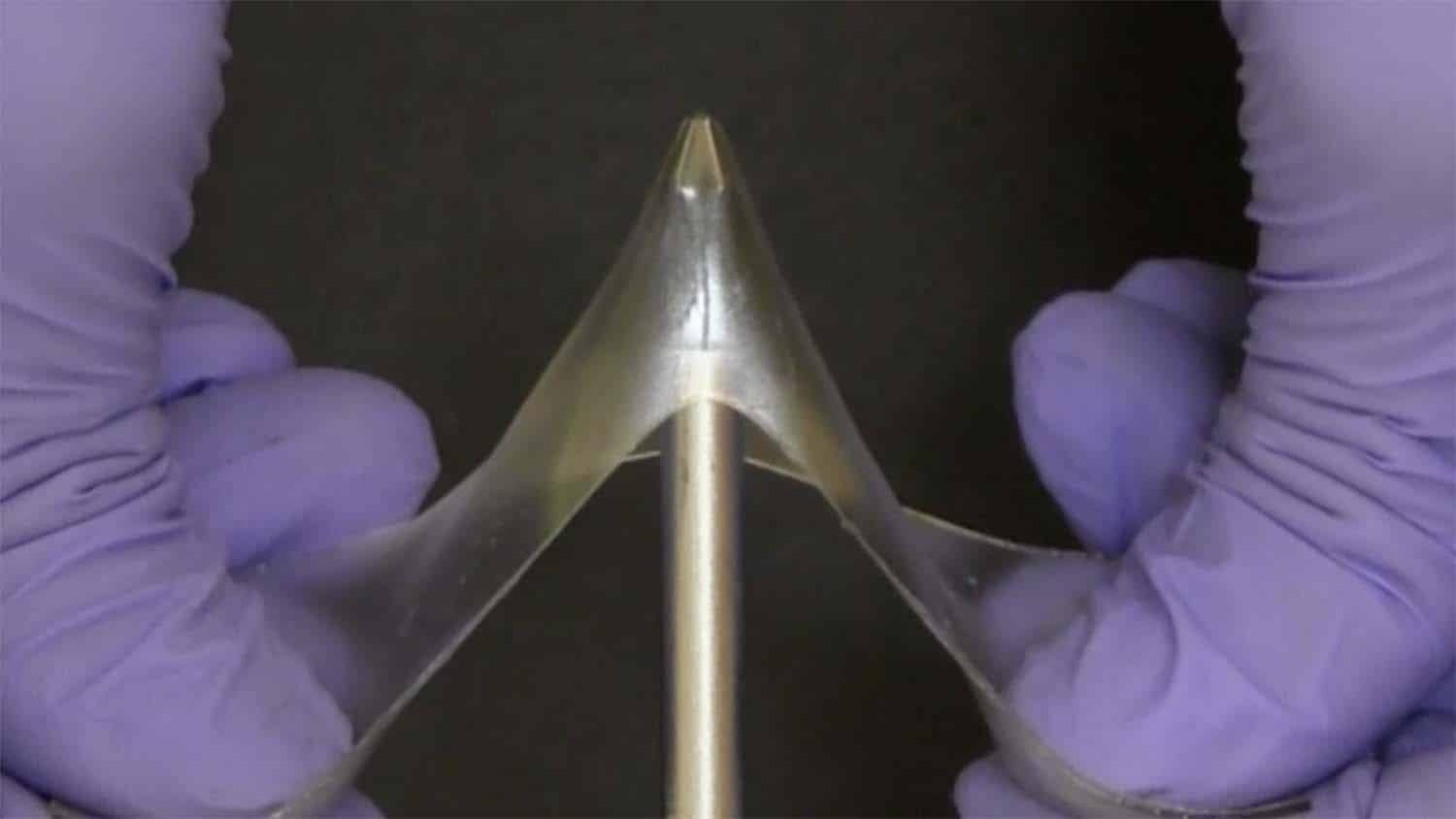
Image Credit: Meixiang Wang.
Gels and glassy polymers are two material classes that were once thought to be separate from one another. Glassy polymers are frequently brittle, stiff, and hard. Items like water bottles and airplane windows are made using it. Gels are soft, stretchable materials that contain liquid, like contact lenses.
We’ve created a class of materials that we’ve termed glassy gels, which are as hard as glassy polymers, but – if you apply enough force – can stretch up to five times their original length, rather than breaking. What’s more, once the material has been stretched, you can get it to return to its original shape by applying heat. In addition, the surface of the glassy gels is highly adhesive, which is unusual for hard materials.
Michael Dickey, Study Corresponding Author and the Camille and Henry Dreyfus Professor, Department of Chemical and Biomolecular Engineering, North Carolina State University
Meixiang Wang, the Study Co-Lead Author and Postdoctoral Researcher at North Carolina State University, said, “A key thing that distinguishes glassy gels is that they are more than 50% liquid, which makes them more efficient conductors of electricity than common plastics that have comparable physical characteristics. Considering the number of unique properties they possess, we’re optimistic that these materials will be useful.”
As the name implies, glassy gels are essentially materials that combine some of the best qualities of gels and glassy polymers. The researchers create them by combining the liquid precursors of glassy polymers with an ionic liquid. The material is "cured" by pouring it into a mold and exposing it to UV light. The glassy gel is then removed along with the mold.
Dickey said, “The ionic liquid is a solvent, like water, but is made entirely of ions. Normally when you add a solvent to a polymer, the solvent pushes apart the polymer chains, making the polymer soft and stretchable. That’s why a wet contact lens is pliable, and a dry contact lens isn’t. In glassy gels, the solvent pushes the molecular chains in the polymer apart, which allows it to be stretchable like a gel.”
However, the ions in the solvent are strongly attracted to the polymer, which prevents the polymer chains from moving. The inability of chains to move is what makes it glassy. The end result is that the material is hard due to the attractive forces, but is still capable of stretching due to the extra spacing.
Michael Dickey, Study Corresponding Author and the Camille and Henry Dreyfus Professor, Department of Chemical and Biomolecular Engineering, North Carolina State University
Although not all polymers can be used to make glassy gels, the researchers discovered that ionic liquids and a wide range of polymers could be used to make glassy gels.
Dickey said, “Polymers that are charged or polar hold promise for glassy gels, because they’re attracted to the ionic liquid.”
According to the researchers, despite being 50–60% liquid, the glassy gels do not evaporate or dry out.
Maybe the most intriguing characteristic of the glassy gels is how adhesive they are. Because while we understand what makes them hard and stretchable, we can only speculate about what makes them so sticky.
Michael Dickey, Study Corresponding Author and the Camille and Henry Dreyfus Professor, Department of Chemical and Biomolecular Engineering, North Carolina State University
As glassy gels are simple to create, the researchers believe the gels have potential for use in real-world scenarios.
Dickey concluded, “Creating glassy gels is a simple process that can be done by curing it in any type of mold or by 3D printing it. Most plastics with similar mechanical properties require manufacturers to create polymer as a feedstock and then transport that polymer to another facility where the polymer is melted and formed into the end product. We’re excited to see how glassy gels can be used and are open to working with collaborators on identifying applications for these materials.”
Xun Xiao from the University of North Carolina at Chapel Hill is one of the paper's co-lead authors. The other co-authors are Salma Siddika, an NC State Ph.D. student; Brendan O'Connor, an NC State Professor of mechanical and aerospace engineering; Wubin Bai, a UNC Professor of applied physical sciences; Ethan Frey, a former NC State undergrad; and Wen Qian, a Research Associate Professor of mechanical and materials engineering at the University of Nebraska–Lincoln.
The Coastal Studies Institute provided funding for a portion of the project.
Glassy Gels Toughened by Solvent
Glassy Gels Toughened by Solvent. Video Credit: North Carolina State University
Journal Reference:
Wang, M., et al. (2024) Glassy gels toughened by solvent. Nature. doi.org/10.1038/s41586-024-07564-0