According to a study published in the Chemical Engineering Journal, scientists at Tokyo Tech discovered that encapsulating copper nanoparticles within hydrophobic porous silicate crystals significantly increased the catalytic activity of copper-zinc oxide catalysts utilized in methanol synthesis through CO2 hydrogenation. The innovative encapsulation structure effectively inhibits the thermal aggregation of copper particles, enhancing hydrogenation activity and increasing methanol production. This breakthrough paves the way for more efficient methanol synthesis from CO2.
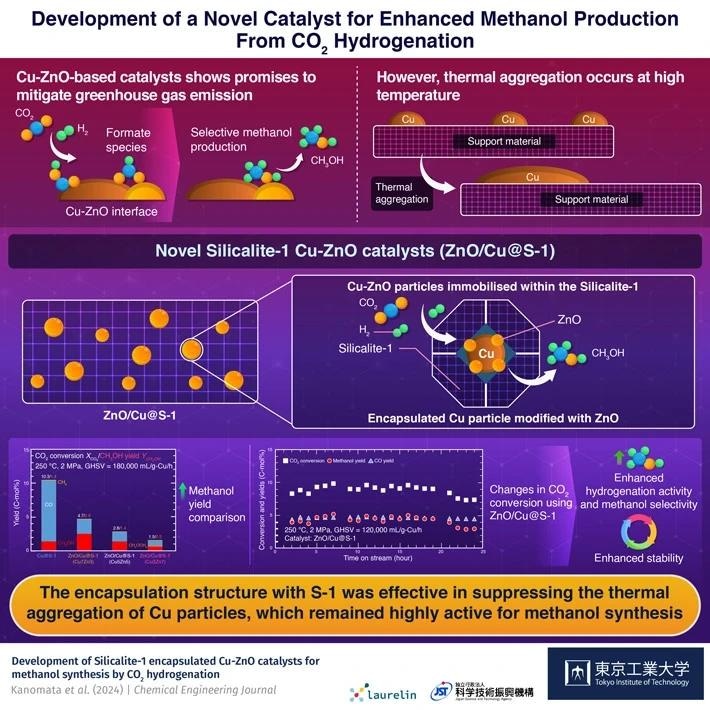
Image Credit: Tokyo Institute of Technology
Carbon dioxide (CO2) emissions are a major contributor to global warming, underscoring the urgent need for emission reduction measures. As a result, there is an increasing exploration of alternatives to fossil fuels, the primary source of CO2 emissions. Methanol emerges as a versatile and cost-effective fuel, offering a promising alternative to conventional transportation fuels.
Additionally, significant attention has been directed towards CO2 capture and utilization technologies to mitigate the impact of these emissions. These innovative approaches involve capturing CO2 from the atmosphere and converting it into value-added products. Among these technologies, methanol synthesis via CO₂ hydrogenation stands out as a particularly promising method.
Lower reaction temperatures are preferred for CO2 hydrogenation-based methanol synthesis since heat is generated throughout the process. As a result, studies have concentrated on the creation of catalysts with high activity at low temperatures.
Copper-zinc oxide (Cu-ZnO)-based catalysts are extremely helpful for this purpose because they can generate a Cu-ZnO interface that binds and transforms CO2 into formate intermediates, which promotes methanol synthesis. Increasing the surface area of this contact is an excellent strategy to boost production, which can be accomplished by increasing the dispersion of Cu nanoparticles.
However, Cu nanoparticles are thermally unstable and tend to aggregate during catalyst preparation and reaction, reducing the interface area. Additionally, the formation of water as a by-product of methanol synthesis accelerates Cu aggregation and inhibits formate formation.
To solve these challenges, a team of researchers led by Professor Teruoki Tago from the Department of Chemical Science and Engineering, School of Materials and Chemical Technology at Tokyo Institute of Technology, created innovative Silicalite-1 (S-1) encapsulated Cu-ZnO catalysts.
Research indicates that encapsulating metals within porous carriers like silica or zeolite effectively mitigates thermal aggregation. Therefore, our focus shifted to developing a novel and efficient Cu-based catalyst for methanol production via CO2 hydrogenation, placing particular emphasis on encapsulating Cu nanoparticles within porous materials.
Teruoki Tago, Professor, Department of Chemical Science and Engineering, School of Materials and Chemical Technology, Tokyo Institute of Technology
The project was financed by the EU’s Horizon2020 Framework and the Japan Science and Technology Agency’s SCICORP (Laurelin Project).
The researchers created two types of catalysts: a Cu/S-1 catalyst, which loaded copper onto hydrophobic S-1 by impregnation, and a Cu@S-1 catalyst, which employed a Cu phyllosilicate (CuPS) powder as a metal source to encapsulate Cu particles in the S-1 zeolite. Cu@S-1 was produced by reducing dissolved CuPS powder.
The researchers looked at the effect of CuPS precursor dissolving time on catalyst characteristics and discovered that incorrect dissolution timings had a substantial impact on Cu particle size.
Optimal precursor dissolution produced a catalyst with about 2.4-nanometer Cu particles enclosed inside S-1, which exhibited optimum catalytic activity. This catalyst exhibited more hydrogenation activity and methanol generation than Cu/S-1.
To increase methanol production, ZnO was infused into Cu@S-1, resulting in a ZnO/Cu@S-1 catalyst with tiny Cu particles. This catalyst showed considerably more activity, indicating the establishment of the Cu-ZnO interface.
Our findings indicate that the encapsulation structure with S-1 effectively suppresses thermal aggregation of Cu particles, while simultaneously facilitating the rapid elimination of the water byproduct from the vicinity of the Cu-ZnO interface, thus enhancing methanol synthesis.
Teruoki Tago, Professor, Department of Chemical Science and Engineering, School of Materials and Chemical Technology, Tokyo Institute of Technology
Overall, this study indicates the efficacy of the novel encapsulation approach for producing highly active catalysts, opening up a new path for efficient methanol generation from CO2.
Journal Reference:
Kanomata, R., et. al. (2024) Development of Silicalite-1 encapsulated Cu-ZnO catalysts for methanol synthesis by CO2 hydrogenation. Chemical Engineering Journal. doi:10.1016/j.cej.2024.149896