Reviewed by Lexie CornerJul 24 2024
In a study published in the journal Angewandte Chemie International Edition, Ritsumeikan University researchers described a photocatalytic process that converts PFAS and other fluorinated polymers (FPs) into fluorine ions at room temperature using visible light.
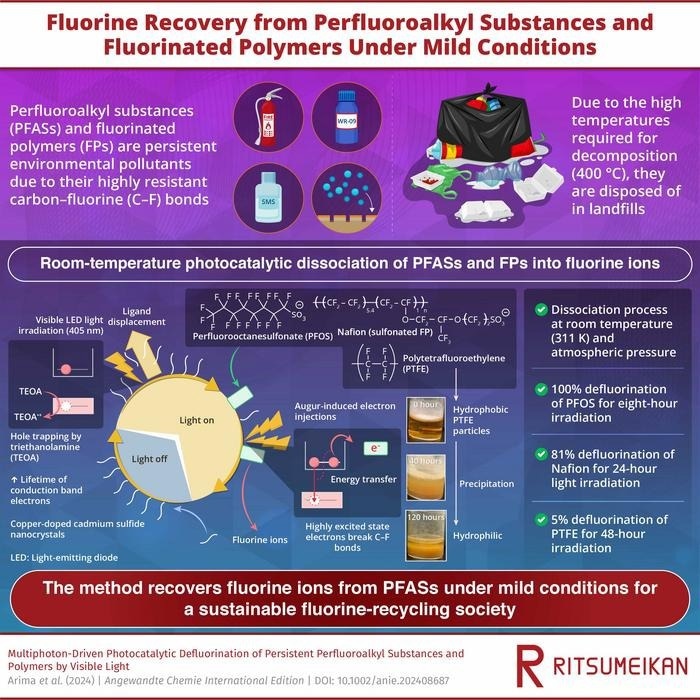 Perfluoroalkyl substances and fluorinated polymers are efficiently decomposed into fluoride ions under ambient conditions by irradiating visible LED light onto semiconductor nanocrystals. The decomposition is driven by cooperative mechanisms involving light-induced ligand displacement and Auger-induced electron injection, utilizing hydrated electrons and higher excited states. Image Credit: Professor Yoichi Kobayashi from Ritsumeikan University, Japan
Perfluoroalkyl substances and fluorinated polymers are efficiently decomposed into fluoride ions under ambient conditions by irradiating visible LED light onto semiconductor nanocrystals. The decomposition is driven by cooperative mechanisms involving light-induced ligand displacement and Auger-induced electron injection, utilizing hydrated electrons and higher excited states. Image Credit: Professor Yoichi Kobayashi from Ritsumeikan University, Japan
Known as “forever chemicals,” perfluoroalkyl substances (PFASs) are an increasing concern to human health and the environment. Due to their remarkable stability and resistance to heat and water, perfluorinated polymers, or PFs, and PFASs have been utilized extensively since the 1938 development of Teflon. These qualities make them perfect for a wide range of uses, such as firefighting foam and cookware.
However, this steadiness has become a significant issue. PFASs are difficult to degrade in the environment, resulting in their buildup in water, soil, and even human bodies. They have been linked to carcinogenesis and hormone disturbance. Today, these chemicals can be detected in drinking water, food, and even Antarctic soil.
Although efforts are being made to phase out PFAS manufacturing, treating them remains difficult since they degrade only at temperatures above 400 °C. As a result, certain products containing PFASs and PFs wind up in landfills, possibly posing future contamination threats.
Researchers at Ritsumeikan University have devised a room-temperature defluorination approach, potentially revolutionizing PFAS treatment.
The researchers used this method to completely defluorinate perfluorooctanesulfonate (PFOS) after just eight hours of light exposure.
The proposed methodology is promising for the effective decomposition of diverse perfluoroalkyl substances under gentle conditions, thereby significantly contributing towards the establishment of a sustainable fluorine-recycling society.
Yoichi Kobayashi, Study Lead Author and Professor, Ritsumeikan University
In the proposed method, visible LED light is irradiated onto cadmium sulfide (CdS) nanocrystals and copper-doped CdS (Cu-CdS) nanocrystals with surface mercaptopropionic acid (MPA) ligands in a solution including PFAS, FPs, and triethanolamine.
The researchers discovered that irradiating these semiconductor nanocrystals produces electrons with a high reduction potential, which breaks the strong carbon-fluorine bonds in PFAS molecules.
To initiate the photocatalytic process, the researchers added 0.8 mg of CdS nanocrystals (NCs), 0.65 mg of PFOS, and 20 mg of TEOA to 1.0 ml of water. They then exposed the solution to a 405 nm LED light.
This light stimulates the nanoparticles, producing electron-hole pairs and driving the removal of MPA ligands from the nanocrystals’ surfaces. This allows PFOS molecules to adsorb onto the NC surfaces.
TEOA is added to catch the holes and extend the lifetime of the reactive electrons available for PFAS breakdown, preventing photoexcited electrons from recombining with holes. Through a process known as Auger recombination, one exciton—an electron-hole pair—recombines with another electron non-radiatively, transferring its energy to produce highly excited electrons.
With adequate energy, these highly excited electrons can engage in chemical interactions with the PFOS molecules adsorbed on the NC surface. Fluorine ions are extracted from the PFAS molecules as a result of the processes that break the carbon-fluorine (C-F) bonds in PFOS.
Laser flash photolysis measurements verified the existence of hydrated electrons produced by Auger recombination by identifying transient species through the absorption spectrum following laser pulse stimulation. The amount of NCs and TEOA employed in the process determined the defluorination efficiency, which rose with the length of light exposure.
The defluorination effectiveness of PFOS was 55 %, 70–80 %, and 100 % for light irradiation lasting one, two, and eight hours, respectively.
Using this strategy, the researchers could defluorinate Nafion, a fluoropolymer, by 81 % after 24 hours of light irradiation. Nafion is commonly used as an ion exchange membrane in electrolysis and batteries.
Fluorine is an essential component in many sectors, including drugs and sustainable energy technologies. Recovering fluorine from waste PFAS can create a more sustainable recycling process and lessen dependency on fluorine production.
Kobayashi concluded, “This technique will contribute to the development of recycling technologies for fluorine elements, which are used in various industries and support our prosperous society.”
Journal Reference:
Arima, Y., et. al. (2024) Multiphoton-driven Photocatalytic Defluorination of Persistent Perfluoroalkyl Substances and Polymers by Visible Light. Angewandte Chemie International Edition. doi:10.1002/anie.202408687