Scientists from the University of Science and Technology of China have created a novel “Janus” dual-atom catalyst (FeCo-N3O3@C), with Fe and Co atoms coordinated synergistically through an N-O bridge, which has demonstrated exceptional performance in both oxygen reduction reaction (ORR) and oxygen evolution reaction (OER). The journal Nature Synthesis published the study.
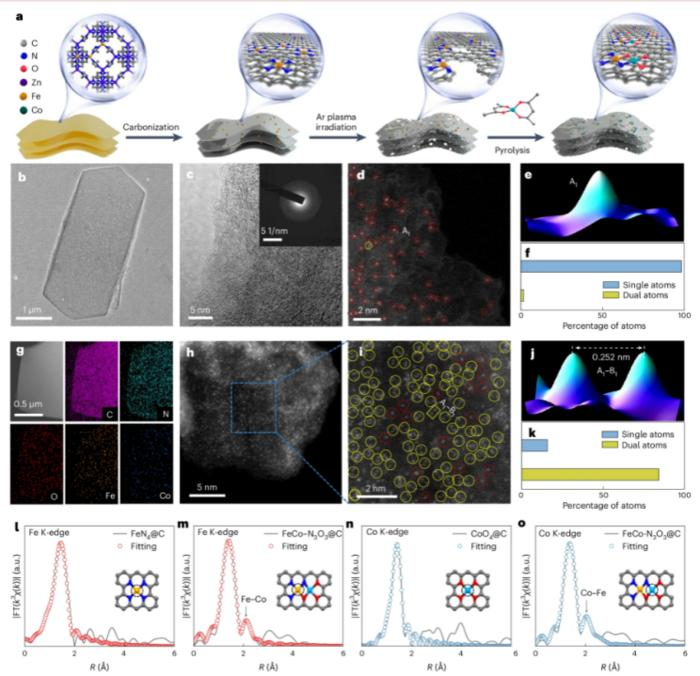 Synthesis Strategy and Structural Characterization of FeCo–N3O3@C Catalyst. Image Credit: Yan et al. University of Science and Technology of China
Synthesis Strategy and Structural Characterization of FeCo–N3O3@C Catalyst. Image Credit: Yan et al. University of Science and Technology of China
Bimetal active-center neighboring atomically distributed diatomic catalysts, which have distinct geometric and electronic structures, demonstrate higher catalytic activity and selectivity than single-atom catalysts. On the other hand, heteronuclear diatomic catalyst multifunctional catalysis and local coordination structure studies are difficult.
Using several ligands, the group created a “double-faced deity” catalyst at the Fe-Co heterobimetallic site. They used spectroscopic methods and multiple scattering theoretical calculations to confirm that the Fe and Co atoms were synergistically coupled with N and O atoms, respectively, through N-O bridging.
According to electrochemical testing, this catalyst performed exceptionally well in both ORR and OER, outperforming the commercial Pt/C+RuO2 combination.
Researchers also discovered that the strong electrical connection between the Fe-N3 and Co-O3 units was responsible for the substance's significant catalytic activity. Subsequent investigation revealed that the strong electronic coupling effect changed the electron density of the d-orbitals of the Fe and Co atoms.
It accelerated the ORR and OER reaction kinetics by optimizing the intermediates' adsorption and desorption processes.
This study presents new insights into the structural characterization and logical design of innovative dual-atom catalysts with outstanding performance.
Journal Reference:
Tang, B., et al. (2024) A Janus dual-atom catalyst for electrocatalytic oxygen reduction and evolution. Nature Synthesis. doi.org/10.1038/s44160-024-00545-1