In a recent study published in the Journal of the American Chemical Society, researchers at Argonne National Laboratory and Ames National Laboratory of the US Department of Energy have discovered an energy-efficient method of producing propylene. They found that when combined with silicon nitride, zirconium enhances propane's catalytic conversion into propylene more efficiently, offering a faster and less energy-intensive alternative to traditional methods.
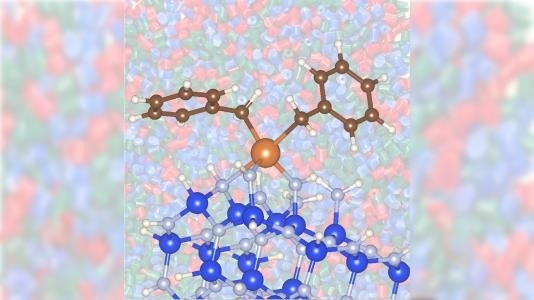 Zirconium (larger burnt orange atom), combined with silicon nitride (blue and gray atoms), enhances the transformation of propane into propylene in a way that's faster and uses less energy than more traditional means. Image Credit: David Kaphan, Max Delferro, and Yu Lim Kim/Argonne National Laboratory
Zirconium (larger burnt orange atom), combined with silicon nitride (blue and gray atoms), enhances the transformation of propane into propylene in a way that's faster and uses less energy than more traditional means. Image Credit: David Kaphan, Max Delferro, and Yu Lim Kim/Argonne National Laboratory
Plastics like polypropylene are widely used in everyday items, including food containers and medical equipment. The chemical needed to create polypropylene is in high demand due to its popularity. Propane can be converted into that chemical, propylene. One natural gas that is frequently utilized in barbecues is propane.
Propane to propylene is usually converted using a metal catalyst (platinum or chromium) on a support (such as silicon dioxide or aluminum oxide). The catalyst accelerates the reaction. However, it also requires high operating temperatures and energy consumption.
Scientists discovered in cooperative research that zirconium and silicon nitride improve propane gas's catalytic conversion to propylene. It accomplishes this more quickly, with less toxicity and energy consumption than other nonprecious metals like chromium. Moreover, its cost is lower than that of platinum and other precious metal catalysts.
This finding also provides a method for lowering the catalytic process's temperature, which lowers the quantity of carbon dioxide released. Nearly 80% of greenhouse gas emissions in the US are caused by carbon dioxide.
Furthermore, this work provides insight into the reactivity that can be attained in the catalytic conversion of propane to propylene using various inexpensive metals.
For some time now, chemists David Kaphan and Max Delferro at Argonne have been methodically examining how unconventional surfaces affect and facilitate catalysis.
As the study's principal investigators, they sought to examine the catalytic conversion of propane between conventionally utilized materials and an unconventional metal catalyst on an unconventional kind of support.
High-surface-area catalyst support materials aid in the dispersion of catalysts. This study indicates that they can also boost catalysis.
The scientists discovered that substantially more active catalysis was produced when using a zirconium catalyst on a silicon nitride substrate to convert propane into propylene. On the other hand, the silica support did not exhibit this behavior.
Additionally, they discovered that catalysis was made possible by the silicon nitride support more quickly and energy-efficiently than by conventional metals on silica. Compared to more commonly utilized oxides, silicon nitride can be used as a catalyst to accelerate chemical reactions on the surface of metals.
Catalytic conversion of propane was accomplished by the scientists at 842 ºF. This is slightly less than the 1022 ºF that is normally needed for catalysis with conventional materials.
Moreover, the reaction rates for this transformation were much faster at the same temperature as conventional catalysts than for comparable materials with oxide supports.
This finding also proves that this idea can be applied to other significant reactions.
This provides a window into nitride-supported metal reactivity. We see promise with the use of other transition metals where we can leverage this difference in the local environment of the nitride surface to enhance catalysis.
David Kaphan, Chemist, Argonne National Laboratory
The Advanced Photon Source (APS) at Argonne, a DOE Office of Science user facility, was helpful to this study. X-ray absorption spectroscopy was employed at beamline 10-BM to help researchers understand how the oxide and nitride materials interact differently with zirconium catalysts.
Ames National Laboratory Scientist Frédéric Perras worked with Argonne scientists to better understand the structure of the zirconium/silicon nitride catalyst. He examined the reaction of silicon nitride with metal sites using dynamic nuclear polarization-enhanced nuclear magnetic resonance.
The composition on the surface of silicon nitride is largely unknown, which is what I found most exciting about this work.
Frédéric Perras, Adjunct Associate Professor, Iowa State University
According to Delferro, this experiment's success was due to the skill of the individuals working on it and the combination of material characterization tools available at Argonne and Ames.
One person cannot do everything. This is really a team effort, and everyone brought their expertise to the table to achieve this goal.
Max Delferro, Chemist, Argonne National Laboratory
Joshua DeMuth, Yu Lim Kim, Jacklyn Hall, Zoha Syed, Kaixi Deng, Magali Ferrandon, A. Jeremy Kropf, and Liu Cong are co-authors.
The study was funded by DOE’s Office of Basic Energy Sciences, Division of Chemical Sciences, Geosciences and Biosciences, Catalysis Science program.
Journal Reference:
DeMuth, J. C., et al. (2024) Silicon Nitride Surface Enabled Propane Dehydrogenation Catalyzed by Supported Organozirconium. Journal of the American Chemical Society. doi.org/10.1021/jacs.4c02776.