Reviewed by Lexie CornerAug 1 2024
A team from the Institute for Solar Fuels at HZB has now studied water splitting at increased pressure under photoelectrochemical (PEC)-relevant circumstances. This study was published in Nature Communications.
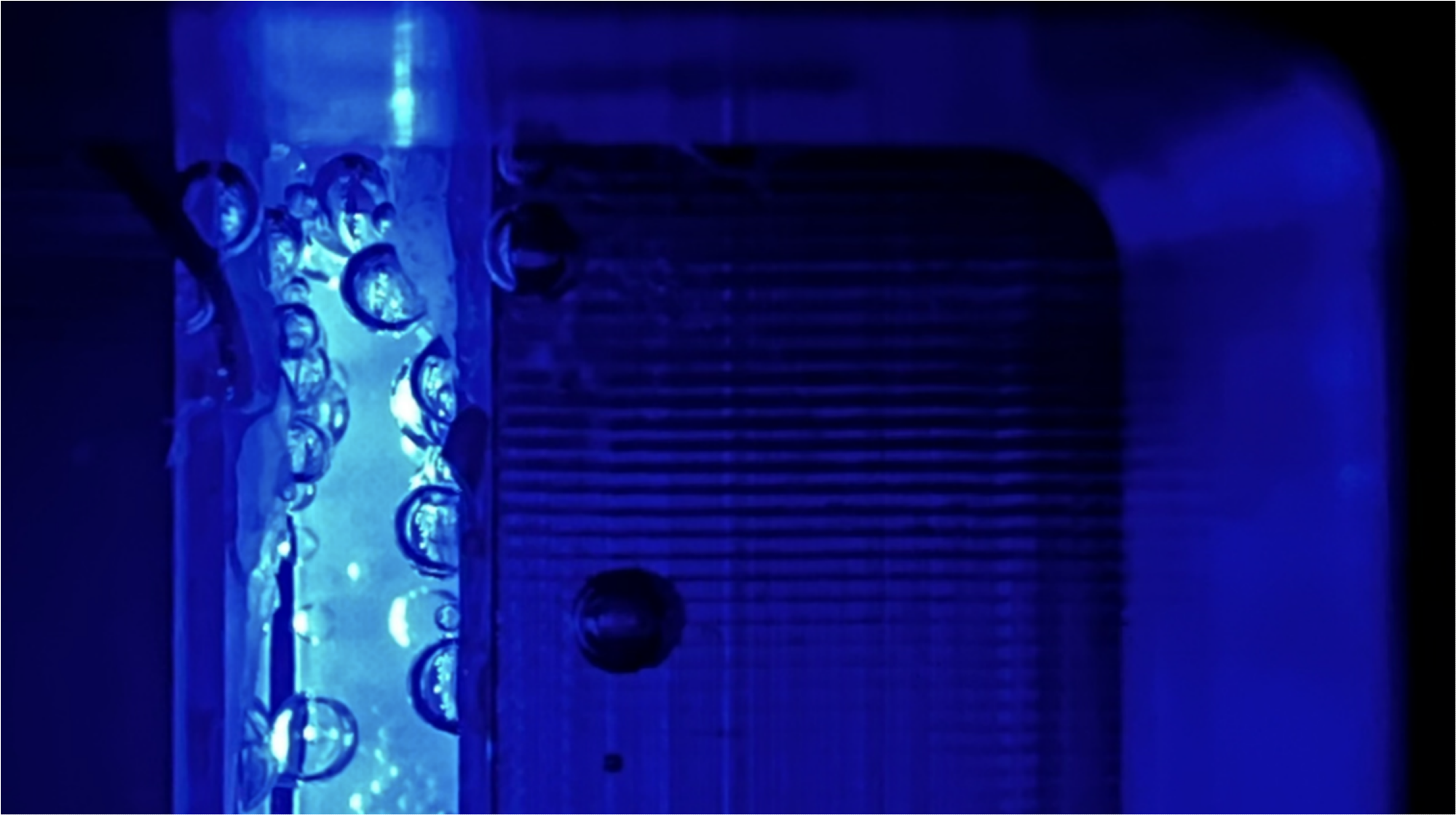 The efficiency of a PEC cell depends on many factors, including the size of the gas bubbles. Image Credit: Feng Liang /HZB
The efficiency of a PEC cell depends on many factors, including the size of the gas bubbles. Image Credit: Feng Liang /HZB
Hydrogen can be created via the electrolytic splitting of water. One alternative is to employ photoelectrodes, which turn sunlight into voltage for electrolysis in photoelectrochemical cells (PEC cells). A research team at HZB has recently demonstrated that the efficiency of PEC cells could be significantly improved under pressure.
Some refer to this as an "artificial leaf" because PEC cells use artificial, inorganic photoelectrodes to generate the voltage needed for the electrolytic splitting of water from sunlight, rather than the natural Photosystem II complex that green leaves use.
Minimizing Losses
The best-performing devices already achieve up to 19 % energy conversion efficiency. At such high efficiencies, losses due to bubble formation begin to play a crucial role. Bubbles scatter light, preventing optimal illumination of the electrode. Bubbles may also block the electrolyte from contacting the electrode surface, thereby causing electrochemical deactivation.
Operating the device at a greater pressure would help lower the diameters of the bubbles, which would minimize these losses. That being said, all PEC devices documented so far have used atmospheric pressure (1 bar).
Enhancing the Pressure
Recently, a group from HZB's Institute for Solar Fuels studied water splitting under high pressure in PEC-relevant circumstances. They collected a variety of characteristics during the electrolysis process and utilized gas to pressurize PEC flow cells to pressures between 1 and 10 bar. A multiphysics model of the PEC process was also created and compared to experimental data obtained at both normal and high pressures.
It is now possible to adjust the settings and locate the important levers with this model.
For example, we investigated how the operating pressure affects the size of the gas bubbles and their behavior at the electrodes.
Dr. Feng Liang, Study First Author and Postdoctoral Researcher, Helmholtz-Zentrum Berlin
Energy Losses Halved
According to the analysis, increasing the operating pressure to 8 bar reduces total energy loss by half, potentially leading to a 5-10 % gain in overall efficiency.
Liang added, “The optical scattering losses can be almost completely avoided at this pressure. We also saw a significant reduction in product cross-over, especially the transfer of oxygen to the counter electrode.”
However, there is no benefit at higher pressures. Hence, the team recommends that the ideal operating pressure range for PEC electrolyzers be between 6 and 8 bar.
These findings, and in particular the multiphysics model, can be extended to other systems and will help us to increase the efficiencies of both electrochemical and photocatalytic devices.
Dr. Roel van de Kro, Materials Scientist, Helmholtz-Zentrum Berlin
The Helmholtz Innopool project “Solar H2: Highly Pure and Compressed” funded this study. The science team acknowledges Torsten Wagner, Lars Drescher, Christian Höhn, and Markus Bürger for their unwavering contributions to the development of this high-pressure flow cell.
Journal Reference:
Liang, F., et. al. (2024) Assessing elevated pressure impact on photoelectrochemical water splitting via multiphysics modeling. Nature Communications. doi:10.1038/s41467-024-49273-2