Reviewed by Lexie CornerAug 21 2024
Researchers at Waseda University have developed a novel one-pot method for converting aromatic ketones into esters, marking a significant advancement in materials science and pharmaceutical synthesis. This innovative approach, published in Chem, opens up new possibilities for utilizing aromatic ketones in various synthetic reactions.
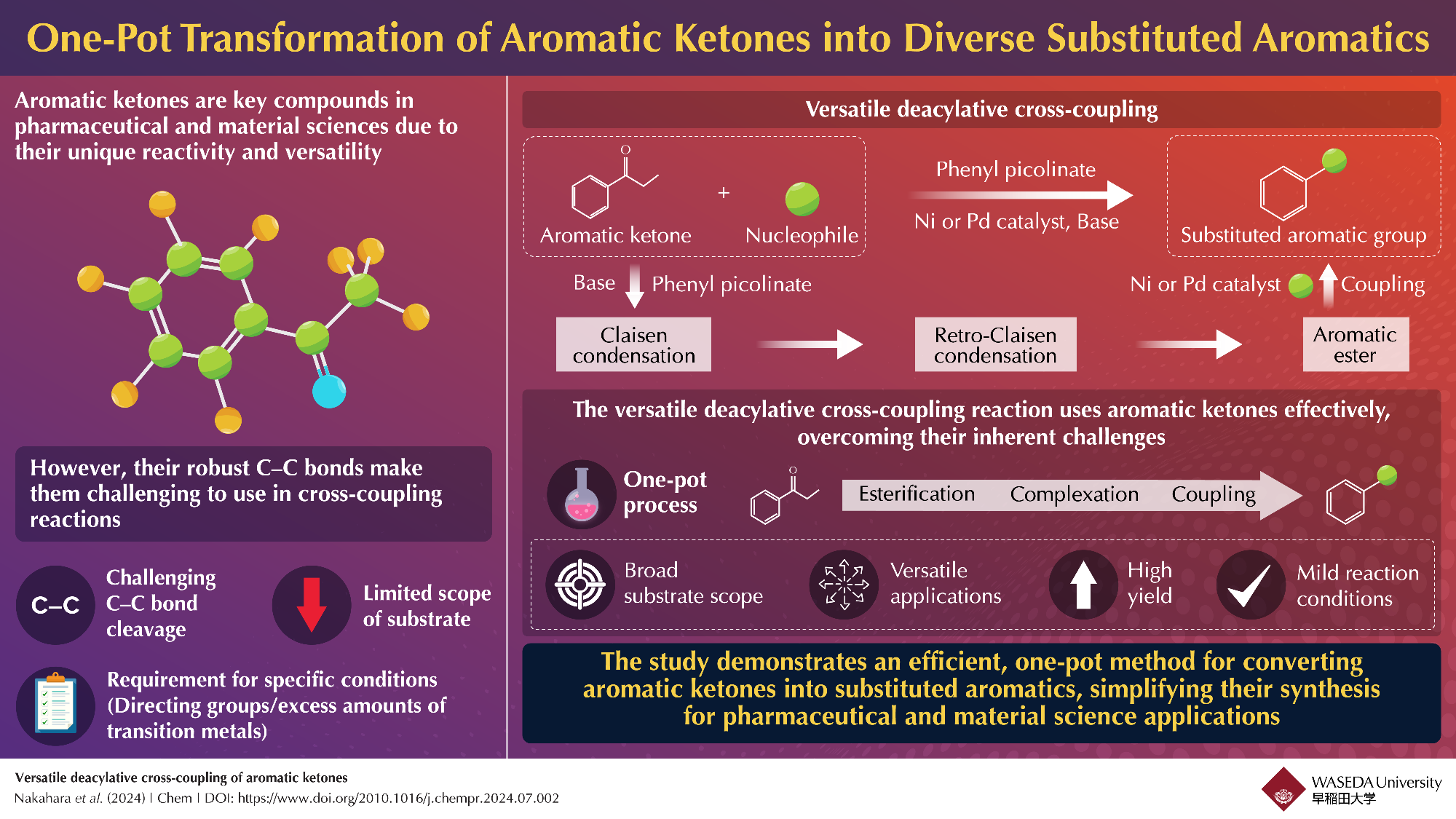
Streamlined One-Pot Conversion of Aromatic Ketones into Aromatic Esters. Researchers have created a simplified one-pot process that converts aromatic ketones into a range of adaptable aromatic esters. Image Credit: Professor Junichiro Yamaguchi from Waseda University.
Traditionally, aromatic ketones have been underutilized in cross-coupling reactions due to the difficulty of breaking their strong carbon-carbon (C–C) bonds. However, researchers have now developed a novel technique that overcomes this challenge by sequentially converting aromatic ketones into aromatic esters through a process that includes a retro-Claisen reaction. These esters can then react effectively with a wide range of nucleophiles, significantly broadening the applications of aromatic ketones in synthesizing valuable aromatic compounds using a one-pot method.
Aromatic ketones have long served as important intermediates in chemical synthesis, particularly in cross-coupling reactions, where they bond with other chemicals to create new compounds. For example, in deacylative cross-coupling, the acyl group is removed from the ketone, enabling the formation of a variety of useful compounds, such as those used in agrochemicals and other industries.
Despite their potential, the strong C–C bonds in aromatic ketones have limited their usefulness because these bonds are difficult to break, often requiring specific conditions or catalysts. Traditional approaches to overcoming this issue have involved complex and costly processes, including the use of directing groups and large amounts of transition metals, which increase the complexity and cost of the procedure, limiting the broader use of aromatic ketones in synthetic and industrial applications.
To address these challenges, a research team led by Professor Junichiro Yamaguchi from Waseda University's Faculty of Science and Engineering, School of Advanced Science and Engineering, has developed a novel one-pot method that optimizes the conversion of aromatic ketones into aromatic esters.
This new method ingeniously combines a Claisen reaction with a retro-Claisen reaction in a single step, greatly simplifying the synthesis process. In the Claisen reaction, two molecules combine to form a larger intermediate compound, which is then converted into the desired ester in the retro-Claisen reaction. By merging these steps into a one-pot process, the researchers have streamlined the synthesis, reduced reaction times, and eliminated the need for additional purification steps, making the process more efficient and practical for wider application.
This advancement enhances the versatility and utility of aromatic ketones, facilitating their use in pharmaceutical synthesis and materials science.
Dr. Junichiro Yamaguchi, Professor and Faculty, Department of Science and Engineering, School of Advanced Science and Engineering, Waseda University
The new reaction method is highly versatile, capable of functioning with a broad range of reactants, including amines, thiols, phenols, and alcohols. It allows for seven distinct types of chemical transformations, converting compounds into thioethers, aryl groups, hydrogen, α-aryls, methylphosphonyl groups, amines, and ethers. This versatility significantly enhances the utility of aromatic ketones across various industries, making the method valuable for a wide array of chemical processes.
Additionally, the catalyst system used in this reaction has shown remarkable stability and reusability, which makes the process scalable for industrial applications.
This reaction represents the first successful example of directly catalyzing aromatic ketones without the use of directing groups, setting the stage for future advancements in synthetic chemistry.
Dr. Junichiro Yamaguchi, Professor and Faculty, Department of Science and Engineering, School of Advanced Science and Engineering, Waseda University
This innovative strategy provides a more efficient, economical, and versatile approach to utilizing aromatic ketones, representing a significant advancement in synthetic chemistry. As this technique is further explored and refined, it has the potential to substantially impact synthetic chemistry and related fields.
Journal Reference:
Nakahara, H., et al. (2024) Versatile Deacylative Cross-coupling of Aromatic Ketones. Chem. doi.org/10.26434/chemrxiv-2024-hd62q