Reviewed by Lexie CornerAug 28 2024
Researchers from the University of Science and Technology of China (USTC) of the Chinese Academy of Sciences (CAS) and the Suzhou Institute for Advanced Study have made a significant breakthrough in water pollution prevention. Their study was published in Nature Communications.
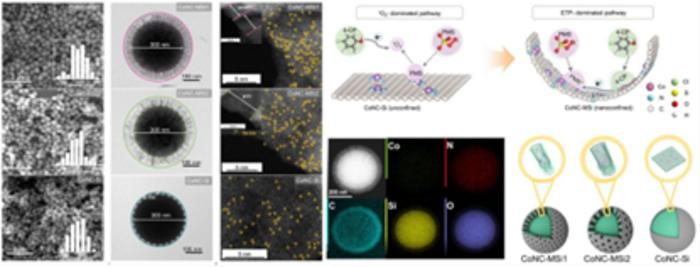 Morphological and Structural Characteristics of Nanoconfined Single-Atom Catalysts and Catalytic Reaction Mechanism. Image Credit: Yan Meng et al.
Morphological and Structural Characteristics of Nanoconfined Single-Atom Catalysts and Catalytic Reaction Mechanism. Image Credit: Yan Meng et al.
The researchers developed a novel method that significantly enhances the efficiency of decomposing contaminants in water by utilizing single-atom catalysts (SACs) within a Fenton-like catalytic system.
Single-atom catalysts are small, potent chemical reaction tools that can aid in water purification by breaking down dangerous contaminants. However, their effectiveness has traditionally been limited by the slow movement of reactants to the catalyst's surface and the large quantities of oxidants required.
Previous studies have linked efficiency increases in nanoconfined SACs to the surface enrichment of pollutants and oxidants. However, the exact mechanisms were not completely known.
The study team found that putting these catalysts inside minuscule, nanometer-sized pores in silica particles could significantly speed up the process and employ oxidants more effectively.
The research team discovered that by confining these catalysts within nanometer-sized pores in silica particles, they could significantly accelerate the reaction process and use oxidants more efficiently. Their research revealed that not only were reactants locally enriched, but the catalytic pathway itself was altered. The process shifted from relying on singlet oxygen, a reactive oxygen species, to a more efficient direct electron transfer method, which is far more effective at breaking down pollutants.
This innovative approach resulted in a remarkable 34.7-fold increase in the rate of pollutant degradation compared to traditional methods. Additionally, the efficiency of oxidant usage improved dramatically, increasing from 61.8 % to 96.6 %.
The system demonstrated remarkable efficacy in breaking down a range of electron-rich phenolic compounds, exhibiting resilience in diverse environmental settings, and sustaining optimal performance in real-world lake water experiments.
The results of this study offer new insights into the functioning of nanoconfined catalysts and present opportunities for the development of effective, low-carbon water purification systems. They also pave the way for future developments in sophisticated oxidation processes and other environmental science applications.
Journal Reference:
Meng, Y., et al. (2024) Nanoconfinement steers nonradical pathway transition in single atom fenton-like catalysis for improving oxidant utilization. Nature Communications. doi.org/10.1038/s41467-024-49605-2