Reviewed by Lexie CornerSep 9 2024
In a study published in the Journal of the American Chemical Society, researchers demonstrated that three-dimensional silicon scaffolds on photoelectrodes enhance the yield of desired products in chemical reactions.
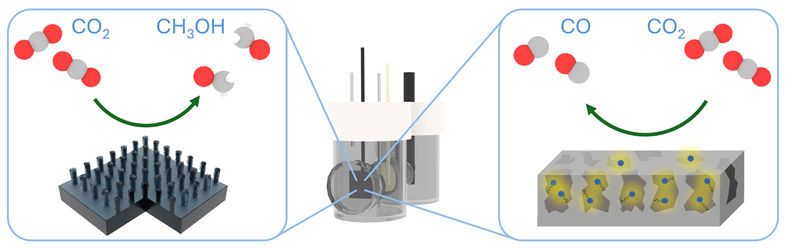 Two examples of silicon photoelectrodes. Left: Micropillar silicon with cobalt catalysts reduces carbon dioxide (CO2) to methanol (CH3OH). Right: Porous silicon with rhenium catalysts reduces carbon dioxide (CO2) to carbon monoxide (CO). Image Credit: Daniel Kurtz, Bo Shang, and Eleanor Stewart-Jones
Two examples of silicon photoelectrodes. Left: Micropillar silicon with cobalt catalysts reduces carbon dioxide (CO2) to methanol (CH3OH). Right: Porous silicon with rhenium catalysts reduces carbon dioxide (CO2) to carbon monoxide (CO). Image Credit: Daniel Kurtz, Bo Shang, and Eleanor Stewart-Jones
Researchers are working to convert carbon dioxide into liquid fuels using light. In the lab, silicon photoelectrodes, combined with light, can transform carbon dioxide into carbon monoxide or methanol. These devices utilize light, including artificial sunlight, to trigger chemical reactions. The incorporation of catalysts into the silicon surface is a crucial part of this process.
The Impact
Researchers have discovered a novel approach to improving the conversion of carbon dioxide into liquid fuel using solar radiation. By incorporating three-dimensional silicon scaffolds onto photoelectrodes, they have successfully increased the yield of desired chemical products.
This technique is particularly noteworthy for its ability to convert carbon dioxide into methanol, which has potential applications as a fuel. Additionally, the process facilitates molecular studies of the catalysts on the photoelectrodes. These photoelectrodes represent a cutting-edge method for producing liquid solar fuel, offering a promising solution for sustainable energy production.
Summary
Although high-surface-area silicon has been known to scientists for many years, it has not been widely used as a light-absorbing semiconductor for liquid fuel production. However, research by the Center for Hybrid Approaches in Solar Energy to Liquid Fuels (CHASE) has demonstrated the significant advantages of employing high-surface-area silicon materials in hybrid photoelectrodes.
One example involves a cobalt catalyst coated on silicon micropillars. This catalyst converts carbon dioxide into methanol while improving current density, resulting in a cutting-edge photoelectrode for generating liquid fuel. In another example, nanoporous silicon was combined with a rhenium catalyst, demonstrating excellent durability and selectivity in converting carbon dioxide to carbon monoxide.
These findings reveal the intrinsic benefits of using high-surface-area silicon semiconductors in hybrid photoelectrodes. This research marks a significant step toward producing liquid fuels from sunlight, using only atmospheric carbon dioxide and water as inputs.
Funding
The study was initially funded through CHASE, an Energy Innovation Hub supported by the Department of Energy (DOE) Office of Science, Office of Basic Energy Sciences. Additional funding for the equipment used in this research came from the DOE and the U.S. National Institutes of Health.
The research also received support from the Air Force Office of Scientific Research and the National Science Foundation. AFM characterization work was funded by the U.S. National Science Foundation and the U.S.-Israel Binational Science Foundation.
Journal References:
Xiaofan, J., et. al. (2024 Photoelectrochemical CO2 Reduction to CO Enabled by a Molecular Catalyst Attached to High-Surface-Area Porous Silicon. Journal of the American Chemical Society. doi.org/10.1021/jacs.3c10837
Bo, S., et. al. (2024) Tailoring Interfaces for Enhanced Methanol Production from Photoelectrochemical CO₂ Reduction. Journal of the American Chemical Society. doi.org/10.1021/jacs.3c13540·
Xiaofan, J., et. al. (2024) Synthesis and Surface Attachment of Molecular Re(I) Complexes Supported by Functionalized Bipyridyl Ligands. Inorganic Chemistry. doi.org/10.1021/acs.inorgchem.2c04137 ·
Bo, S., et. al. (2024) Aqueous Photoelectrochemical CO2 Reduction to CO and Methanol over a Silicon Photocathode Functionalized with a Cobalt Phthalocyanine Molecular Catalyst. Angewandte Chemie. doi.org/10.1002/ange.202215213·