Reviewed by Danielle Ellis, B.Sc.Sep 10 2024
In a study published in the Journal of the American Chemical Society, researchers at the Department of Energy's Oak Ridge National Laboratory discovered a chemical “chameleon” that could improve the process of purifying rare-earth metals used in clean energy, medicine, and national security applications.
 Conceptual art shown here depicts a ligand adapting to its environment. ORNL scientists have discovered a compound that shifts its preference for binding to specific rare-earth metals depending on surrounding conditions, like a chameleon changes its colors. Image Credit: Adam Malin/ORNL, U.S. Dept. of Energy
Conceptual art shown here depicts a ligand adapting to its environment. ORNL scientists have discovered a compound that shifts its preference for binding to specific rare-earth metals depending on surrounding conditions, like a chameleon changes its colors. Image Credit: Adam Malin/ORNL, U.S. Dept. of Energy
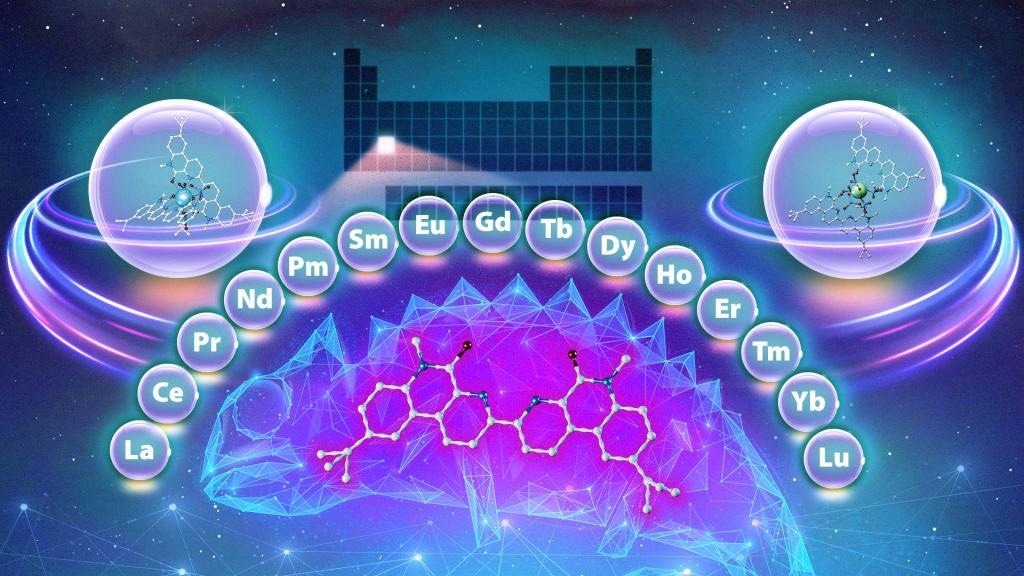
The study, conducted in collaboration with Vanderbilt University, is the latest in a series of efforts by ORNL’s Chemical Sciences Division to reduce barriers to accessing metals known as lanthanides, which are widely used in a variety of products and applications, including biomedical imaging, industrial chemical production, and electronics. The rare-earth metals consist of 15 lanthanides and two additional elements.
Contrary to their name, most rare-earth metals are not uncommon; lanthanides exist naturally in mineral ore deposits, and several are as prevalent in the environment as copper and lead
However, the metals’ tremendous qualities that make them so widely utilized are only useful if an individual lanthanide is extracted from the combination of other metals in which it is found when mined. The metal of choice must be highly refined before it can be used in its intended use. The complexity of this technique is what makes it rare.
It is a big challenge because the lanthanide ions are very similar in their sizes and chemical properties. They differ by only the slightest amount, so isolating pure individual lanthanides requires very precise separations science.
Subhamay Pramanik, R&D Associate Staff Scientist, Chemical Sciences Division, Oak Ridge National Laboratory, Oak Ridge National Laboratory
To isolate a specific metal from rare-earth mineral solutions, scientists and industry utilize ligands, which are chemical compounds that preferentially bind to a certain metal in the solution. These chemicals are dissolved in an organic solvent before being combined with a water-based solution of the lanthanide combination.
Organic solvents, like oil, do not combine with water, allowing the layers to separate. If the molecule effectively extracts the target metal from the solution during mixing, it draws it into the organic layer as the solvent and aqueous solution separate. The metal can then be further treated and refined.
The best current commercial separation procedures are staged, with lanthanides separated in a certain order—heavy to light or light to heavy. The procedure is time-consuming and expensive, and it generates a significant amount of trash that is not necessarily environmentally friendly.
Enter the chameleon. When scientists investigated an existing ligand identical to the compounds employed in the aforementioned method, they discovered something novel: a ligand that behaves differently depending on the experimental settings.
The compound, which acts like a chameleon, alters its behavior in response to its surroundings, binding to various lanthanides depending on the acid content of the solution and the length of time the ligand is allowed to interact with it. For example, if the environment is acidic, the ligand will preferentially bind to a heavier lanthanide.
In typical separation systems, a ligand usually shows preference for either lighter or heavier lanthanides. We found you can use the same compound to perform multiple different separations, which is exciting and unique. And we identified the mechanisms by which it does it.
Santa Jansone-Popova, Study Co-Lead and Synthetic Organic Chemist, Oak Ridge National Laboratory
Using the same compound to separate numerous lanthanides in the series may reduce the number of steps required in this typical and costly operation. Furthermore, depending on the circumstances, the ligand used in this study might separate the heaviest, lightest, and mid-weight lanthanides in any order.
Other ligands do not exhibit the same behavior. Until recently, scientists had no idea this one would either. The chameleon ligand looks like other well-known ligands; however, it behaves radically differently. Now that it is known that such capabilities and systems exist for lanthanide-binding compounds, the ligand can be examined in greater detail, and other compounds with similar features can be found.
Just because the structure of a ligand looks very similar to another, it doesn’t have to behave the same, and that understanding moves the needle and pushes the boundaries of what’s known. It has the potential to make the separations processes faster, cleaner, and better — reducing the number of stages, providing better selectivity and purity, and leading to more environmentally friendly processes.
Ilja Popovs, Study Co-Lead and R&D Staff Member, Oak Ridge National Laboratory
Using the same compound to separate numerous lanthanides in the series may reduce the number of steps required in this common and costly operation. Furthermore, depending on the circumstances, the ligand used in this work might separate the heaviest, lightest, and medium-weight lanthanides in any order.
The DOE's Office of Science Separation Science and Materials Chemistry programs funded this study. Parts of the investigation used two DOE Office of Science user facilities: Argonne National Laboratory's Advanced Photon Source and Brookhaven National Laboratory's National Synchrotron Light Source II.
Journal Reference:
Pramanik, S., et. al. (2024) Tetradentate Ligand’s Chameleon-Like Behavior Offers Recognition of Specific Lanthanides. Journal of the American Chemical Society. doi.org/10.1021/jacs.4c07332