Reviewed by Danielle Ellis, B.Sc.Sep 10 2024
Associate Professor Takahiro Ohkubo of the Graduate School of Engineering at Chiba University, along with his research team comprising Taiki Uno from the same university, Professors Ippei Maruyama and Naohiko Saeki from The University of Tokyo, Associate Professor Yuya Suda from University of Ryukyus, Atsushi Teramoto from Hiroshima University, and Professor Ryoma Kitagaki from Hokkaido University, examined the mechanism of carbonation reaction under various Ca/Si ratios and relative humidity conditions in a study published in The Journal of Physical Chemistry C on July 24th, 2024.
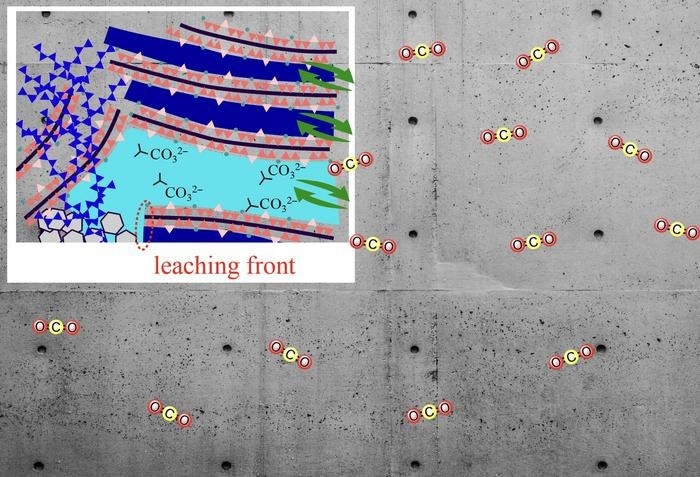
The interplay of water transport and related structural changes in cement-based materials help absorb atmospheric carbon dioxide in the form of CO32-. Image Credit: Takahiro Ohkubo from Chiba University
One of the main contributors to global warming at the moment is carbon dioxide (CO2) emissions. Through a process known as carbonation, cement-based materials have demonstrated promising uses in capturing and solidifying CO2 as minerals, providing a potential remedy to lessen the effects of climate change. As a result, a great deal of research has been done to increase the carbonation efficiency of materials based on cement.
In short, the process of carbonation in cement paste is the dissolution of CO2 in water, which is followed by an interaction with C-S-H, hydrates that are created when raw materials are hydrated. In the process, the dissolved CO2 produces carbonate ions (CO32-), which then combine with the calcium ions (Ca2+) from C–S–H to produce precipitates of calcium carbonate. However, since the cement paste components are unstable, the whole explanation of carbonation processes remains unclear despite several research studies conducted in varied settings.

Image Credit: Guenter Albers/Shutterstock.com
Previous studies have demonstrated that the following factors significantly affect carbonation: the concentration and saturation level of water in C-S-H, the calcium/silicate (Ca/Si) ratio, relative humidity (RH), and CO2 solubility. Furthermore, it is critical to comprehend how ions and gel-pore water—water that passes through the nanopore-sized pores in C-S-H layers—affect this process.
The role of water transport and carbonation-related structural changes remains an open question. In this study we used a new method to study these factors, using 29Si nuclear magnetic resonance (NMR) and 1H NMR relaxometry, which has been established as an ideal tool for studying water transport in C–S–H.
Takahiro Ohkubo, Associate Professor, Graduate School of Engineering, Chiba University
The scientists created C-S-H and used 100% CO2, which is significantly more than ambient levels, to accelerate the carbonation process and analyze it.
Ohkubo added, “Natural carbonation in cement materials occurs over several decades by absorbing atmospheric CO2, making it difficult to study in a lab setting. Accelerated carbonation experiments with elevated CO2 concentrations provide a practical solution to this challenge.”
The samples were created with different Ca/Si ratios and RH levels. They also used 1H NMR relaxometry to study the water exchange mechanisms in a deuterium dioxide (D2O) environment and 29Si NMR to study the C–S–H samples.
The Ca/Si ratio of the C-S-H chain and the RH had a significant impact on the structural alterations brought about by the carbonation reaction, which included the collapse of the C-S-H chain structure and modifications in pore size. Furthermore, smaller-sized pores were produced via lower RH and a higher Ca/Si ratio. This prevented water and Ca2+ ions from leaking from the interlayer gap into gel pores, which resulted in ineffective carbonation.
Ohkubo stated, “Our study shows that the carbonation process occurs due to a combination of structural modifications and mass transfer, signifying the importance of studying their interplay, rather than just structural changes.”
Further emphasizing the significance of the present study, Associate Professor Ohkubo added, “Our findings can contribute to developing new building materials that can absorb large amounts of atmospheric CO2. Additionally, carbonation reactions are also common in organic matter and hence, our new approach will also help to understand the carbonation of compounds in the natural environment.”
To sum up, this research provides insight into the carbonation response of cement-based materials and suggests a possible way to reduce CO2 emissions.
Journal Reference:
Uno, T., et al. (2024) Understanding the Carbonation Phenomenon of C–S–H through Layer Structure Changes and Water Exchange. The Journal of Physical Chemistry C. doi.org/10.1021/acs.jpcc.4c01714.