Reviewed by Danielle Ellis, B.Sc.Sep 10 2024
Researchers from Kyushu University have shown that a zeolite material called Na-ZSM-5 is useful in enhancing the chemical conversion of biomass into olefins—a precursor chemical that is used to make everything from plastics to pharmaceuticals—using microwaves. Their study was published in the Chemical Engineering Journal.
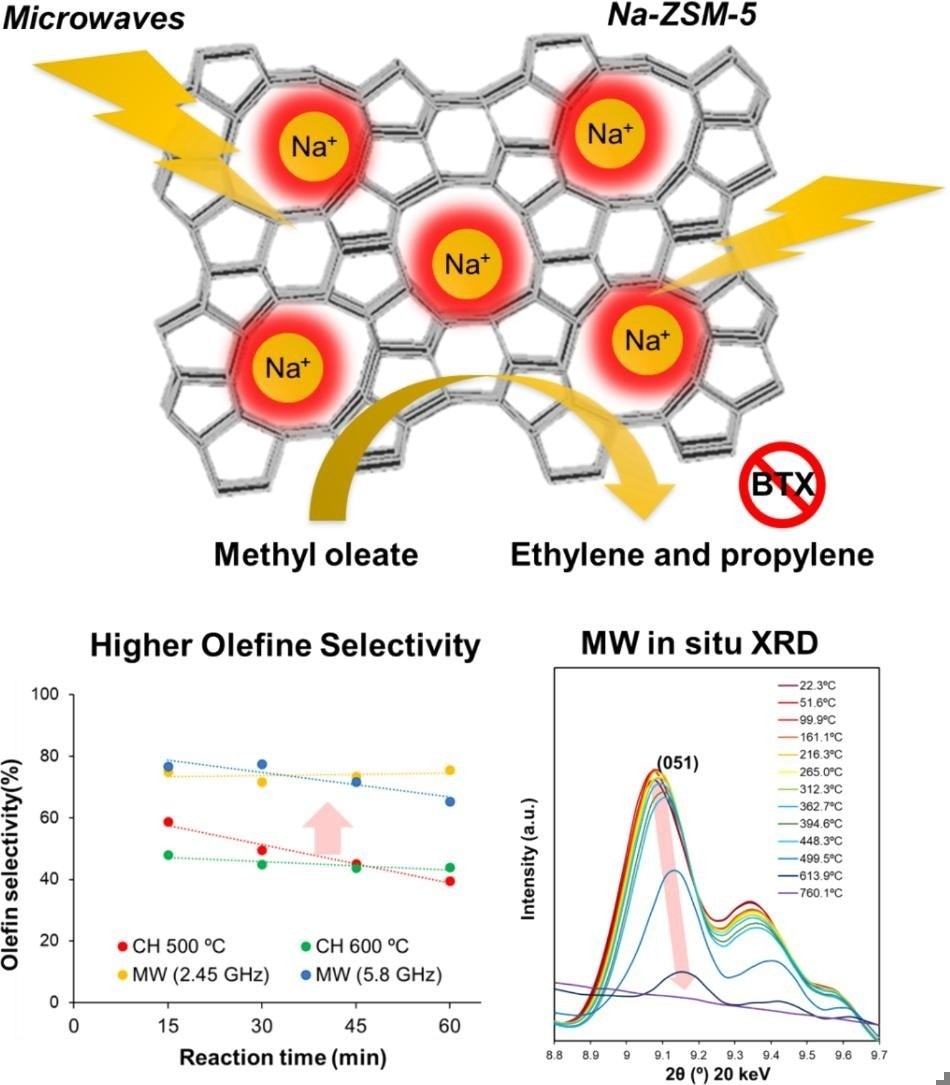
Design and performance of the proposed microwave-heatable catalyst. Researchers from Kyushu University used microwaves to heat up a sodium-substituted zeolite catalyst, Na-ZSM-5, so that it could more efficiently convert biomass to useful industrial precursor chemicals. without harmful side effects. Image Credit: Kyushu University/Shuntaro Tsubaki
The group argues that heating Na-ZSM-5 in a microwave might lead to a more sustainable and energy-efficient chemical sector.
Chemical precursors with simple structures are usually the first step in the synthesis of complex organic compounds, such as food additives, drugs, and plastics. A great deal of study has been done on effectively and sustainably synthesizing precursor chemicals.
One often employed technique for producing these necessary chemicals is the reforming of naphtha. Nevertheless, this process emits carbon dioxide and uses a lot of energy. Microalgal oils and cooking oil waste have been proposed as low-cost alternatives for synthesizing these basic chemicals.
Zeolite is a material that can be used in a process known as “catalytic cracking” to convert these oils. Zeolite is a naturally occurring porous material that is frequently employed as an absorbent or catalyst. Up to 500–600 °C of heat is required for the materials to undergo catalytic cracking. Operating at such temperatures can result in an accumulation of undesirable deposits, a process known as coking, which shortens the catalyst's lifetime in addition to being extremely energy-intensive.
In the current study, associate professor Shuntaro Tsubaki of the Faculty of Agriculture at Kyushu University and his colleagues conducted microwave experiments to heat zeolite catalysts to the necessary temperature without causing side effects like coking.
Microwaves interact directly with materials and can selectively deliver energy to them, enabling significant energy savings compared to conventional heat-convective processes. In particular, microwaves can accelerate gas–solid catalysis by passing straight through the gas phase and selectively heating the solid catalyst. They achieve this by forming spatial hot spots inside the catalyst bed.
Shuntaro Tsubaki, Associate Professor, Faculty of Agriculture at Kyushu University
In their study, the researchers first tested numerous zeolite catalysts to see which ones could be successfully heated by microwaves while still providing high catalytic activity. After conducting both theoretical and experimental evaluations, they settled on Na-ZSM-5, a sodium ion-substituted zeolite.
To demonstrate the benefits of microwave heating over traditional heating, the researchers performed a catalytic conversion of methyl oleate. When microwave heating was applied, Na-ZSM-5 outperformed other catalysts, producing high conversion efficiency of fatty acid esters into olefins while maintaining excellent selectivity. Furthermore, carbon dioxide generation was limited to 1.3% of the overall reaction output, with no carbon monoxide created at all.

Image Credit: Martin Mecnarowski/Shutterstock.com
Most significantly, microwave heating of Na-ZSM-5 to 500 °C produced four times more olefin than traditional heating to 500 °C. This was partly due to Na-ZSM-5's better selectivity for producing olefins rather than other compounds. Furthermore, coke production was not detected when microwave heating was utilized, even at temperatures as high as 600 °C.
Finally, the researchers examined the local structural alterations in the zeolite upon exposure to microwaves to provide insight into why microwave heating enhanced certain parts of the catalytic process.
It is interesting to note that even while the bulk material’s temperature stayed around 500 °C, they discovered that microwave absorption led to localized temperatures of over 1000 °C in the zeolite’s crystal lattice. The formation of olefins was probably selectively driven by these extremely high temperatures.
Catalysts heated in a microwave might greatly increase catalytic biomass conversion and aid in the current chemical industry's pursuit of sustainability.
Tsubaki added, “Our findings are expected to contribute to the further electrification of the chemical industry. Since microwaves can be generated from renewable energy sources such as solar and wind, we can reduce the environmental impact of the synthesis of these fundamental chemicals.”
The researchers intend to optimize microwave-driven catalytic processes further, with the goal of increasing yield and energy efficiency while expanding capacity. They think that their efforts will usher in a new age of sustainable chemical production.
Journal Reference:
Ota, S., et al. (2024) Microwave-enhanced catalytic conversion of fatty acid ester to olefins by Na-ZSM-5. Chemical Engineering Journal. doi.org/10.1016/j.cej.2024.154737.