Reviewed by Danielle Ellis, B.Sc.Sep 16 2024
In a recent study published in the journal ACS Energy Letters, researchers developed a lithium-sulfur (Li-S) battery with an enhanced iron sulfide cathode to solve stability and safety concerns. Two prototypes show remarkable stability over 300 cycles of charge and discharge, and one prototype continues to function even after being sliced or folded.
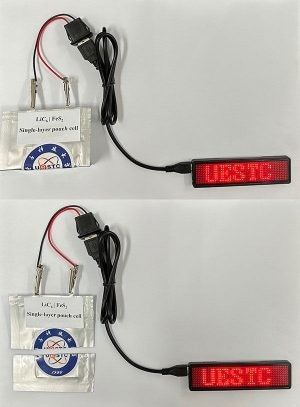 This lithium-iron sulfide battery pouch cell can be folded (top image) or cut (bottom image) and still provide power. Image Credit: Adapted from ACS Energy Letters
This lithium-iron sulfide battery pouch cell can be folded (top image) or cut (bottom image) and still provide power. Image Credit: Adapted from ACS Energy Letters
Lithium-ion technology is used in the majority of rechargeable batteries that power portable electronics like e-bikes, toys, and pocket vacuums. However, these batteries have a limited lifespan and can catch fire if they sustain damage.
Due to its inexpensive cost and ability to store more energy than lithium-metal oxides and other materials used in conventional ion-based versions, sulfur has been proposed as a material for lithium-ion batteries. Researchers have previously suggested separating the two electrodes of Li-S batteries, an iron sulfide cathode, and a lithium metal-containing anode, using a carbonate-based electrolyte to make the batteries stable at high temperatures.
However, the cathode's sulfide quickly loses capacity in the cell because it dissolves into the electrolyte and forms an impermeable precipitate. Liping Wang and associates pondered whether it would be possible to lessen corrosion without compromising functioning or rechargeability by introducing a layer between the cathode and electrolyte.
In the first electrochemical performance tests, the researchers coated iron sulfide cathodes in several polymers and discovered that polyacrylic acid (PAA) performed the best, maintaining the electrode's discharge capacity after 300 charge-discharge cycles.
The researchers then integrated a graphite-based anode, a carbonate-based electrolyte, a lithium metal foil serving as an ion source, and a PAA-coated iron sulfide cathode into a prototype battery architecture. They created and tested prototype batteries for coin cells and bag cells.
No significant capacity loss in the pouch cell was noticed by Wang and colleagues after more than 100 charge-discharge cycles. Further tests revealed that the pouch cell remained functional even after it was folded and sliced in half. The coin cell held onto 72% of its capacity even after 300 cycles of charge and discharge.
They then coated different metal cathodes with the polymer to create lithium-vanadium and lithium-molybdenum batteries. Over 300 charge-discharge cycles, the cells' capacity remained consistent as well. According to Wang's team, the overall results show that coated cathodes could generate efficient batteries with various metal sulfides in addition to safer Li-S batteries with long lifespans.
The research was supported by the National Natural Science Foundation of China, the Natural Science Foundation of Sichuan, China, and the Beijing National Laboratory for Condensed Matter Physics.
Journal Reference:
Liu, H., et al. (2024) Chelating-Type Binders toward Stable Cycling and High-Safety Transition-Metal Sulfide-Based Lithium Batteries. ACS Energy Letters. doi.org/10.1021/acsenergylett.4c01907.