Reviewed by Danielle Ellis, B.Sc.Sep 17 2024
An international group of researchers from the University of Pennsylvania found that liquid crystals can condense into amazing structures under appropriate circumstances. They can form filaments and flattened discs that move materials around, much like intricate biological systems. This research was published in the journal Proceedings of the National Academy of Sciences.
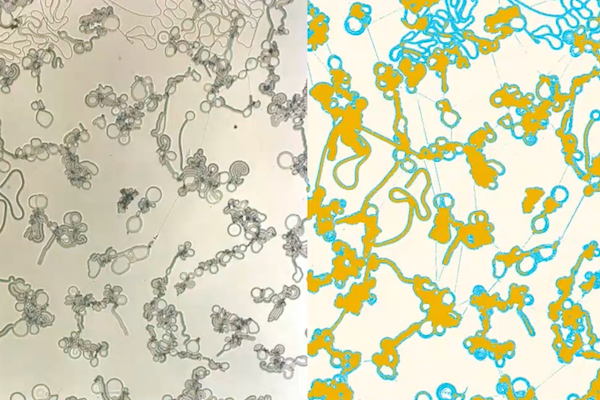 Under the right conditions, liquid crystals form astonishing structures reminiscent of biological systems, shown here in actual (left) and false color (right), with the filaments in light blue and the flattened discs in yellow. Image Credit: Christopher Browne
Under the right conditions, liquid crystals form astonishing structures reminiscent of biological systems, shown here in actual (left) and false color (right), with the filaments in light blue and the flattened discs in yellow. Image Credit: Christopher Browne
Liquid crystals are found in everything from automobile dashboards to medical equipment to mobile phone screens and video game consoles. Liquid crystal displays (LCDs) produce color when an electric current is sent through them because of two special characteristics of the fluids: they can change shape and reflect various light wavelengths.
This discovery could result in new approaches to modeling biological activity, self-assembling materials, and more.
It is like a network of conveyor belts. It was this serendipitous observation of something that superficially looks very lifelike that was the initial cue that this might be something more general and more interesting.
Christopher Browne, Postdoctoral Researcher and Study Co-First Author, University of Pennsylvania
Now, Browne and Osuji are a part of an NSF-funded multidisciplinary group at the Laboratory for Research on the Structure of Matter (LRSM) studying condensate formation in biological and non-biological systems under the direction of Elizabeth Rhoades, a Professor of Chemistry in the School of Arts & Sciences, and Matthew Good, an Associate Professor of Cell and Developmental Biology at the Perelman School of Medicine.
Osuji’s lab collaborated with ExxonMobil to research mesophase pitch, a material used in producing high-strength carbon fibers found in Formula 1 cars and premium tennis rackets.
Those materials are liquid crystals, of the chemical precursors to the carbon fibers themselves. Or better stated, they are liquid crystalline over some period of their existence during processing.
Chinedum Osuji, Eduardo D. Glandt Presidential Professor and Chair, Chemical and Biomolecular Engineering, University of Pennsylvania
Yuma Morimitsu, another postdoctoral scholar at the Osuji Lab and co-first author of the paper, observed peculiar behavior in the condensates during experiments at various temperatures.
Generally, when two immiscible (i.e., non-mixable) fluids are combined and heated to a temperature that forces them to mix, the combination will eventually separate, or "demix," if it is cooled. This usually occurs through the creation of droplets that eventually combine to form a distinct layer, much like when water and oil are combined, and the result is an oil layer on top of the water.
In this instance, the liquid crystal 4'-cyano 4-dodecyloxybiphenyl, or 12OCB, separated from the colorless oil squalane by spontaneously forming extremely irregular patterns.
Instead of forming drops, when you have this phase separation between the liquid crystal and the other components of the system, you form cascaded structures, the first of which is these filaments, which grow rapidly and thereafter form another set of structures — what we call bulged discs or flat droplets.
Chinedum Osuji, Eduardo D. Glandt Presidential Professor and Chair, Chemical and Biomolecular Engineering, University of Pennsylvania
The movement of the liquid crystals was observed by the researchers using sophisticated microscopes to gain an understanding of the system at the micrometer scale, which is millionths of a meter, or the breadth of a human hair.
“The first time we saw these structures, we looked at them at a cooling rate that was excessively high,” recalled Osuji, leading the liquid crystals to clump together.
The liquid crystals were building structures that resembled biological systems, but the researchers did not discover this until they reduced the cooling rate and increased the magnification.
It is interesting to note that, as Browne discovered, several researchers had nearly observed comparable activity decades before, but they either examined systems in which the behavior was not very noticeable or lacked powerful enough microscopy to see what was truly happening.
The most intriguing aspect of the result, according to Browne, is how it unites several previously unrelated fields: phase behavior and self-assembly, which study materials that self-assemble into new structures and exhibit unique phase behavior, and active matter research, which studies biological systems that move matter and generate motion.
“This is a new type of active matter system,” said Browne.
He and Osuji further suggest that the discovery may be used to mimic biological systems to produce materials or better understand how they function.
Osuji said, “Molecules are being absorbed into the filaments and then shuttled into those flat droplets continuously, even though just by looking at the system, you cannot discern any obvious activity.”
The flat droplets might behave as miniature reactors, agitating molecules that are then transported by the filaments to other droplets for storage or additional chemical reactions.
Additionally, the researchers propose that their discovery might stimulate further investigation into liquid crystals.
Browne said, “When a field becomes industrialized, oftentimes the fundamental research tapers off. But sometimes there are lingering puzzles that nobody finished solving.”
This research was conducted at the University of Pennsylvania, in the School of Engineering and Applied Science’s Department of Chemical and Biomolecular Engineering, the School of Arts & Sciences’ Department of Physics and Astronomy, and ExxonMobil’s Research Division. ExxonMobil and the US National Science Foundation supported the study.
Zhe Liu of Penn Engineering; Paul G. Severino of the School of Arts & Sciences; and Manesh Gopinadhan, Eric B. Sirota, Ozcan Altintas, and Kazem V. Edmond of ExxonMobil are the Co-authors of the research.
Journal Reference:
Morimitsu, Y., et al. (2024) Spontaneous assembly of condensate networks during the demixing of structured fluids. Proceedings of the National Academy of Sciences. doi.org/10.1073/pnas.2407914121.