Reviewed by Danielle Ellis, B.Sc.Oct 7 2024
Researchers at the Universities of Bonn and Montreal have created a novel kind of catalyst to create methane from carbon dioxide and water as efficiently as possible with electricity. Methane is used for various purposes, such as starting material in the chemical industry and apartment heating. The research was published in the journal Nature Chemistry.
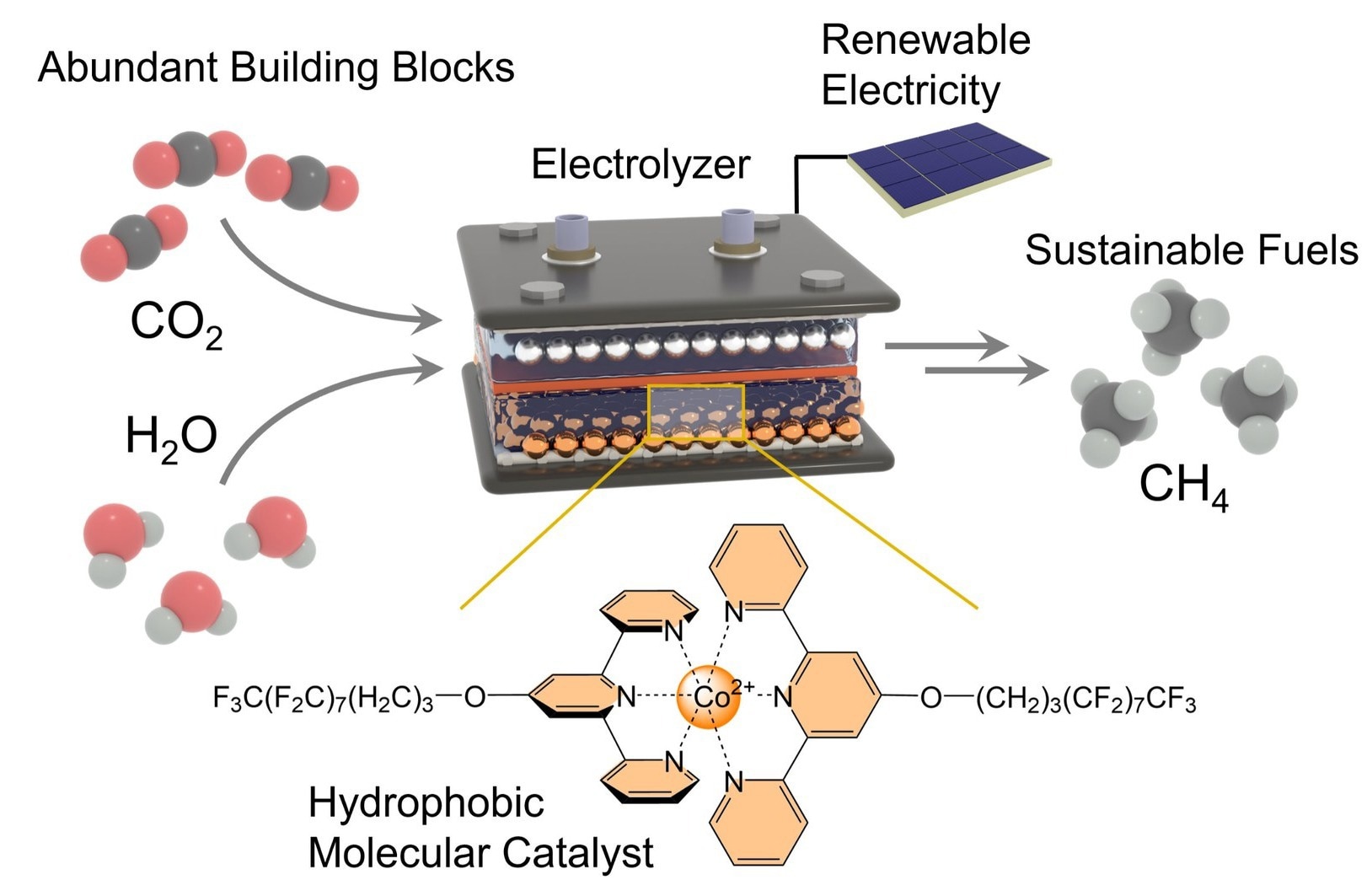
It is the primary component of natural gas and, when produced using green electricity, is largely climate-neutral. The researchers' understanding of the model system they examined can be applied to large-scale technological catalysts. It is possible to generate other significant chemical compounds using this technique.
Energy is needed for many chemical reactions to begin, and this energy can be given by heating or applying high pressure to the reaction partners, for example.
We used electricity as the driving force instead. By using climate-friendly electricity, we can produce, for example, methane that does not contribute to global warming.
Dr. Nikolay Kornienko, Professor, University of Bonn
The researcher moved from the University of Montreal to the Institute of Inorganic Chemistry at the University of Bonn. He initiated his most recent study while in Canada and completed it after relocating to his new institution.
“The production of methane – which has the chemical formula CH4 – is challenging because it is necessary to carry out a reaction between a gas and a liquid,” says Kornienko.
The study discusses water (H2O) and carbon dioxide (CO2). The researchers brought these two partners together using a gas diffusion electrode. The reaction requires separating the two oxygen atoms (chemical symbol: O) from the carbon atom (C) and substituting them with four hydrogen atoms (H). Water is the source of the hydrogen.
Preventing Side Reactions
The issue with this approach is that water prefers to go through a different reaction and will instantly split into hydrogen and oxygen when it comes into contact with an electric current.
This is a competing reaction that we have to avoid. Otherwise, it would stop us from producing any methane. Therefore, we have to prevent the water from coming into contact with the electrode. At the same time, we still need the water as a reaction partner.
Morgan McKee, Institute of Inorganic Chemistry, University of Bonn
This is the function of the recently created catalyst, which is applied to the electrode. Above all, it ensures that carbon dioxide reacts faster and makes it easier to form methane. It does this by weakening the bonds that bind the carbon atom to the two oxygen atoms and containing the carbon dioxide in its so-called “active center.”
The following step involves gradually substituting four hydrogen atoms for these oxygen atoms. At this point in the process, the catalyst requires water. However, it must also maintain a safe distance to prevent any unwanted side effects.
Kornienko said, “To achieve this, we bound long molecular side chains to the active center. Their chemical structure repels water or, in other words, they are hydrophobic.”
Professor Kornienko is also a member of the Transdisciplinary Research Area “Matter” at the University of Bonn.
Water-Fearing Molecular Chains
This specialized phrase translates to “having a fear of water” and is derived from Greek. The side chains serve as a kind of conveyor belt, keeping the H2O molecules away from the electrode and active center. In other words, they grab hydrogen atoms from the water molecules and move them to the active core, where they combine with the carbon atom. In this fashion, CO2 is transformed into CH4 in numerous phases.
This reaction creates almost no unwanted side products, and the process is efficient over 80%. Nevertheless, the catalyst is not truly ideal for the large-scale generation of methane.
The reaction principles we have achieved with this catalyst could, however, be realized in other catalyst materials for use in large-scale technical applications.
Dr. Nikolay Kornienko, Professor, Universities of Bonn
The researcher thinks there are other uses for this technique besides the production of methane. He believes that it may be more profitable to produce other chemical compounds, such as ethylene, which is the raw material for many plastics. Hence, in the medium run, it might be possible to reduce the environmental impact of plastic manufacture by using the novel catalyst method whenever feasible.
The investigation had participation from the following institutions: The Universities of Bonn, Montreal (Canada), Swansea (Wales), Bayreuth, Oulu (Finland), Hohenheim, FU Berlin, and the Synchroton SOLEIL in Saint-Aubin (France).
The research was supported by the Natural Sciences and Engineering Research Council of Canada (NSERC), the Engineering and Physical Sciences Research Council (EPSRC), the Higher Education Funding Council for Wales (HEFCW), and the Erasmus+ program from the EU.
Journal Reference:
McKee, M., et al. (2024) Hydrophobic assembly of molecular catalysts at the gas–liquid–solid interface drives highly selective CO2 electromethanation. Nature Chemistry. doi.org/10.1038/s41557-024-01650-6.