Reviewed by Danielle Ellis, B.Sc.Oct 8 2024
A group of researchers from Tohoku University, Tokyo University of Science, and Mitsubishi Materials Corporation have made a significant advancement in increasing the efficiency of the photocatalytic reaction that converts water into hydrogen, according to a study published in the Journal of the American Chemical Society.
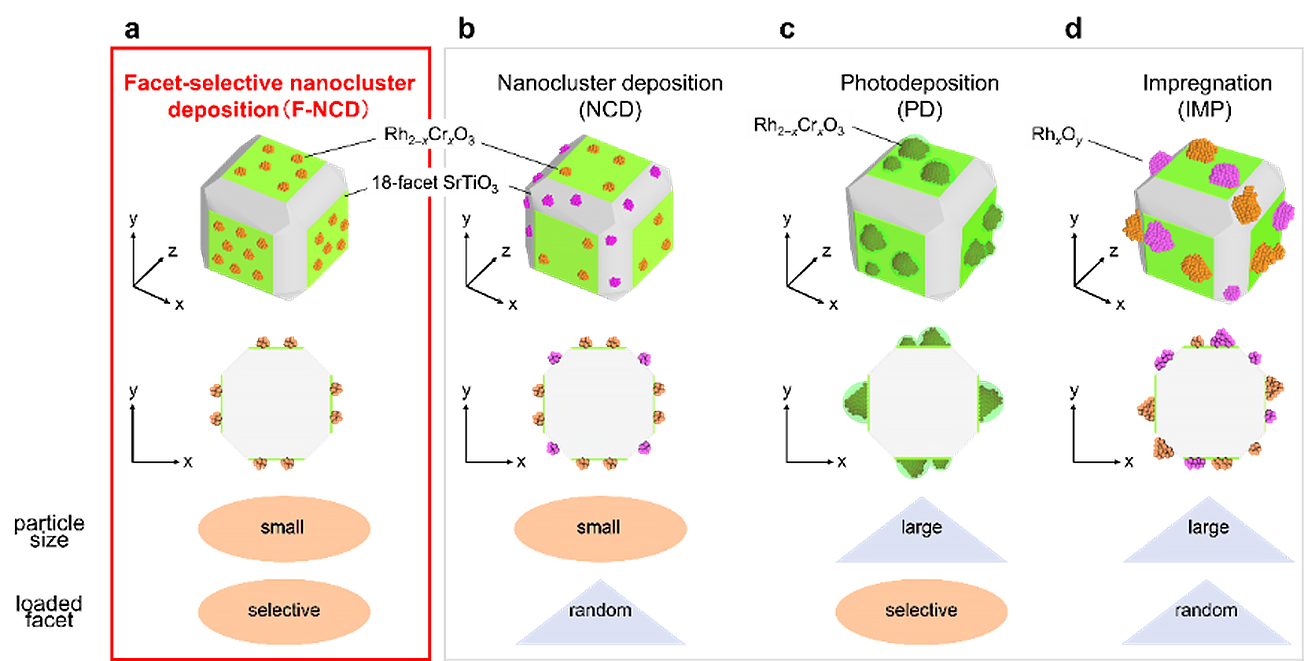 (a) developed crystal facet-selective loading method of nanocluster (F-NCD; this method), (b) conventional nanocluster deposition (NCD), (c) photoelectrodeposition (PD), and (d) impregnation (IMP) method. Image Credit: Yuichi Negishi et al.
(a) developed crystal facet-selective loading method of nanocluster (F-NCD; this method), (b) conventional nanocluster deposition (NCD), (c) photoelectrodeposition (PD), and (d) impregnation (IMP) method. Image Credit: Yuichi Negishi et al.
In an urgent effort to become carbon neutral, scientists are looking for clean fuel sources like hydrogen. Researchers from Tokyo University of Science, Tohoku University, and Mitsubishi Materials Corporation have made a significant advancement in the efficiency of the photocatalytic reaction that converts water into hydrogen.
Water-splitting photocatalysts can produce hydrogen (H2) from only sunlight and water. However, the process hasn't been optimized sufficiently for practical applications. If we can improve the activity, hydrogen can be harnessed for the realization of a next-generation energy society.
Yuichi Negishi, Study Lead Researcher and Professor, Tohoku University
Using ultrafine rhodium (Rh)-chromium (Cr) mixed-oxide (Rh2–xCrxO3) cocatalysts (the actual reaction site and a crucial component to stop H2 reforming with oxygen to make water again) with a particle size of roughly 1 nm, the research team developed a novel technique.
They are then selectively loaded onto a photocatalyst, which accelerates reactions using water and sunlight. Prior research has not achieved these two achievements in a single reaction—a small cocatalyst that can also be applied to particular areas of the photocatalyst—.
A smaller particle size is crucial because it increases the cocatalyst’s specific surface area, which significantly boosts activity per quantity of cocatalyst loaded. Additionally, facet-selective loading is crucial because if it is not, cocatalysts positioned at random may wind up on crystal facets where the intended reaction does not take place.
In the photocatalyst made using the F-NCD method (Rh2-xCrxO3/18-STO (F-NCD)), the cocatalyst's particle size, loading position, and electronic state were compared to those made using the traditional method.
In photocatalysts made using the new technique, water-splitting photocatalytic activity was increased by 2.6 times overall. The resulting photocatalyst shows the highest apparent quantum yield for strontium titanate obtained thus far.
Thanks to this amazing technique, the ability to produce hydrogen without producing toxic byproducts like carbon dioxide has improved. This could help us all breathe a little easier by enabling us to use hydrogen as a more numerous, environmentally friendly energy source.
Journal Reference:
Hirayama, D. et. al. (2024) Ultrafine Rhodium–Chromium Mixed-Oxide Cocatalyst with Facet-Selective Loading for Excellent Photocatalytic Water Splitting. Journal of the American Chemical Society. doi.org/10.1021/jacs.4c07351