Reviewed by Danielle Ellis, B.Sc.Oct 8 2024
According to a study that was published on October 7th, 2024, in the Journal of the American Chemical Society, researchers from Yokohama National University aim to lessen the environmental impact of the chemical manufacturing sector by concentrating on renewable energy sources and alternative techniques for producing the chemical building blocks of some of the most widely used compounds.
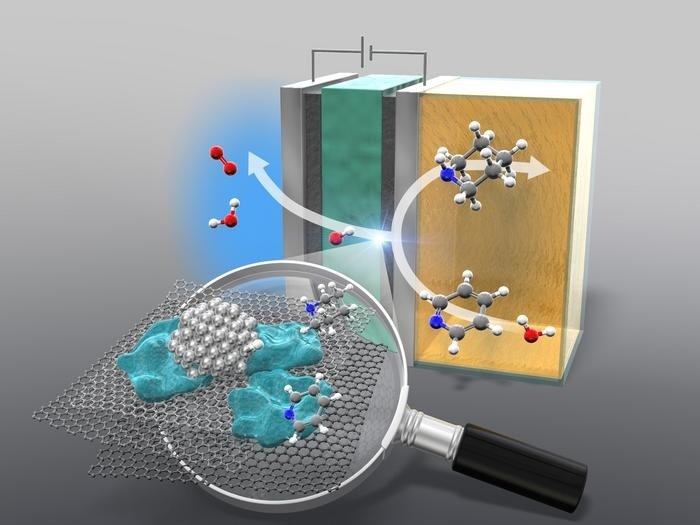 The anion-exchange membrane reactor hydrogenates pyridine to piperidine. Image Credit: Yokohama National University
The anion-exchange membrane reactor hydrogenates pyridine to piperidine. Image Credit: Yokohama National University
Finding a more environmentally friendly method of producing the chemical building blocks for commonly used and widely consumed compounds is essential to successfully reducing the environmental impact of the chemical manufacturing sector.
It is no secret that manufacturing processes have some of the most severe and profound effects on the environment; the manufacturing of chemicals leads the way in terms of emissions and energy consumption. Although this makes sense, given the extensive use of manufactured chemicals in daily life, there is still much room for improvement in terms of sustainability.
Cyclic amines are the primary subject of this study since they are the most crucial building blocks for fine chemicals. These compounds contain a nitrogen atom and are arranged in a ring. The cyclic amine piperidine, which is crucial to the fine chemical industry, is derived from pyridine, one of the main stars of the show.
For instance, piperidine serves as the building block for numerous materials, including pesticides, FDA-approved drugs, and commonplace items used in the daily lives of many people.
Typical methods for adding hydrogen to a nitrogen-containing cyclic amine include using hydrogen gas as a proton and electron source. The hydrogenation process uses hydrogen obtained from the steam reforming of methane, a major greenhouse gas.
Not only is this method energy intensive, but it also accounts for approximately 3% of global carbon dioxide emissions. This process is also highly dependent on fossil fuels and requires a significant amount of energy. Fortunately, researchers have developed an anion-exchange membrane (AEM) electrolyzer to address this issue.
An AEM electrolyzer allows for the hydrogenation of various types of pyridines at ambient temperature and pressure, eliminating the need for acidic additives as in traditional methods. The electrolyzer works by splitting water into its constituents, atomic hydrogen and oxygen. The atomic hydrogen obtained is then mixed into the cyclic compound.
The AEM electrolyzer is also extremely versatile with other nitrogen-containing aromatics, making it a promising candidate for a variety of applications. Furthermore, by developing a method that can be used at normal temperatures and pressures, the amount of electrical energy required for the process is significantly reduced.
The method offers significant potential for industrial-scale applications in pharmaceuticals and fine chemicals, contributing to the reduction of carbon emissions and advancing sustainable chemistry.
Naoki Shida, Study First Author and Researcher, Yokohama National University
Unlike the conventional method, which relies on fossil fuels, this process uses water and renewable electricity as an energy source. This method has not compromised efficiency, and the percent yield on a large scale is 78%, demonstrating that this technology is reasonably scalable.
One issue that may arise during the electrolysis process is an increase in cell voltage; however, this can be mitigated by either improving AEM or, preferably, designing an AEM with organic electrosynthesis in mind.
For electrocatalytic hydrogenation technology to gain traction and make a difference, it must be scalable on an industrial scale so that pharmaceutical and fine chemical companies can use it. The more this technology is used, the easier it is to adapt it to other nitrogen-containing aromatic compounds, demonstrating the feasibility of the electrocatalytic hydrogenation process.
Ideally, this method would establish itself as a viable alternative to traditional methods used in the chemical industry, lowering the overall carbon footprint of chemical manufacturing.
Naoki Shida, Mahito Atobe, Yugo Shimizu, Akizumi Yonezawa, Juri Harada, Yuka Furutani, and Yusuke Muto of the Department of Chemistry and Life Science at Yokohama National University with Naoki Shida and Mahito Atobe also of the Institute of Advanced Sciences at Yokohama National University contributed to this study.
Naoki Shide of PRESTO at the Japan Science and Technology Agency, Ryo Kurihara and Kazuhide Kamiya of the Research Center for Solar Energy Chemistry at Osaka University, Junko N. Kondo of the Institute of Innovative Research at the Tokyo Institute of Technology, Eisuke Sato, Koichi Mitsudo and Seiji Suga of the Division of Applied Chemistry at Okayama University, Shoji Iguchi of the Graduate School of Engineering at Kyoto University, and Kazuhide Kamiya of the Innovative Catalysis Science Division at Osaka University were also a part of this study.
Journal Reference:
Shida, N. et. al. (2024) Electrocatalytic Hydrogenation of Pyridines and Other Nitrogen-Containing Aromatic Compounds. Journal of the American Chemical Society. doi.org/10.1021/jacs.4c09107