Researchers at Osaka University have discovered a method to create durable, high-performance polymers—the primary building block of plastics—that can be precisely and easily broken down into their constituent parts and recycled into nearly new materials. The study was recently published in Chemical Science.
Plastics are essential to many aspects of modern life; without them and their beneficial qualities, areas like technology, medicine, and food safety would not exist. However, plastics’ toughness—which is frequently desired—also makes them hazardous pollutants and challenging to recycle. Making it simpler to recycle plastics is the answer to this significant and expanding issue.
Polymers are long chains of small repeating units called monomers that make up the majority of plastics. The recycled plastic is typically worse than the original, and current physical recycling merely reuses the polymers without degrading them.
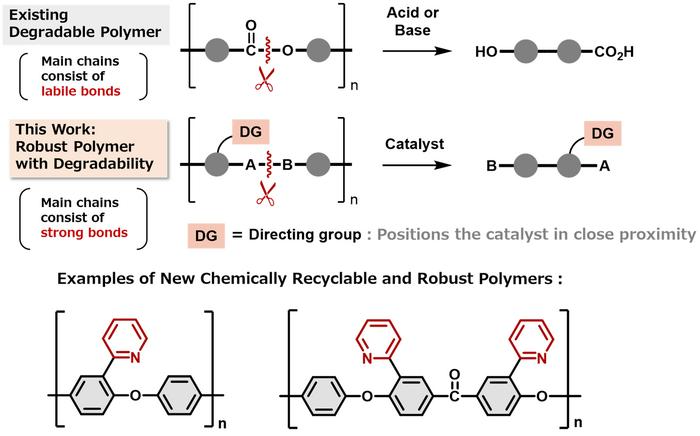
A more recent technique is chemical recycling, which disassembles polymer chains into their monomer units and then reassembles the units. The repurposed plastic is brand new. However, because weak bonds between the monomer units make it simple to break the chains apart, polymers intended for chemical recycling are typically weak.
Without sacrificing heat and chemical resistance, the researchers have created a method for creating strong, chemically recyclable polymers. This discovery could substantially expand the applications of chemically recyclable polymers.
We knew that we needed to make the links between the monomers really strong in harsh environments but easily broken under specific conditions for recycling. We were surprised to find that no one had tried including a directing group, which would break the strong links only in the presence of a metal catalyst.
Satoshi Ogawa, Study Lead Author, Faculty of Medicine, Osaka University
Similar to a lock on a link, the directing group only opens the link when the appropriate key is present. The polymers resisted harsh chemicals and high temperatures, but a nickel catalyst played a crucial role in recycling, allowing the directing group to easily open the links and liberate the monomers. The monomers could then be put back together to form the original polymer.
It is a huge step forward to make a polymer this tough that can be broken down easily and precisely and recycled into a pristine material in so few steps. This revolutionary design could be used in making high-performance polymers that can be recycled indefinitely with no loss of quality.
Mamoru Tobisu, Study Senior Author and Professor, Osaka University
The team’s efforts demonstrate that performance and recyclability don't have to be mutually exclusive. To make many types of plastic chemically recyclable, their design could be applied to numerous other polymers, which could help put plastic pollution in the trash cans of the past.
Journal Reference:
Ogawa, S. et. al. (2024) Controlled degradation of chemically stable poly(aryl ethers) via directing group-assisted catalysis. Chemical Science. doi.org/10.1039/D4SC04147J