Reviewed by Lexie CornerNov 18 2024
In a study published in Energy & Environmental Science, Dr. Hyung-Suk Oh and Dr. Woong Hee Lee from the Clean Energy Research Center at the Korea Institute of Science and Technology (KIST, President Sang-Rok Oh) created a silver-silica composite catalyst that can control local pH using a silica-hydroxide cycle.
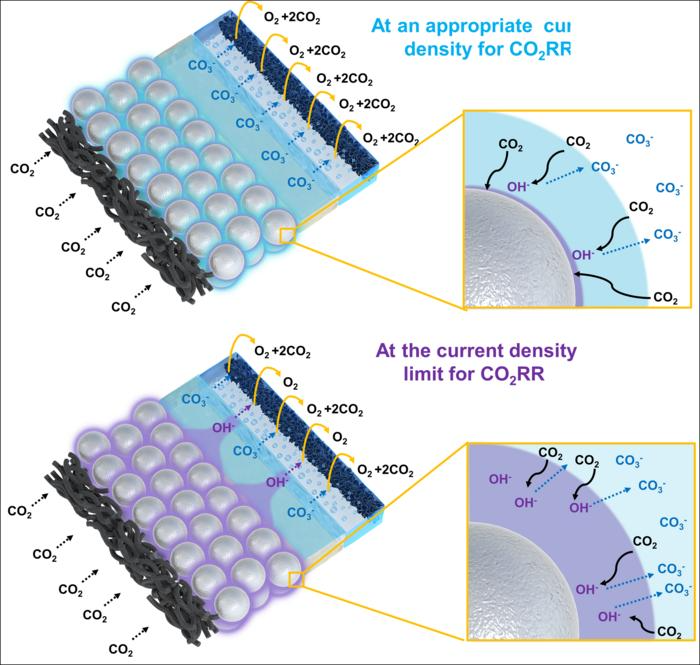 The upper figure depicts the CO₂ reduction reaction at an appropriate current density, while the lower figure illustrates the CO₂ reduction reaction at a high current density in a diagrammatic form. In the CO₂ reduction reaction, hydroxide ions (OH⁻) are generated more abundantly at high current densities, combining with CO₂ and preventing its transfer to the catalyst surface, which reduces performance. Image Credit: Korea Institute of Science and Technology
The upper figure depicts the CO₂ reduction reaction at an appropriate current density, while the lower figure illustrates the CO₂ reduction reaction at a high current density in a diagrammatic form. In the CO₂ reduction reaction, hydroxide ions (OH⁻) are generated more abundantly at high current densities, combining with CO₂ and preventing its transfer to the catalyst surface, which reduces performance. Image Credit: Korea Institute of Science and Technology
The study is based on the carbonate-silicate cycle, also known as the Earth's inorganic carbon cycle, where carbon dioxide (CO₂) maintains equilibrium. In this cycle, volcanic activity releases CO₂ back into the atmosphere after it is extracted from the air and deposited in weathered minerals.
The weathering of silicate rocks produces dissolved silica (SiO₂), which leads to the formation of carbonate rocks. These rocks are later recycled back into silicate rock by volcanic activity, influencing the regulation of Earth’s temperature. Silica, a key component of this cycle, has been used in electrochemical CO₂ conversion processes.
Silver catalysts, which are used in Carbon Capture and Utilization (CCU) technology, are highly effective in converting CO₂ into CO, a valuable raw material for petrochemical products. However, silver catalysts are not yet commercially viable because they suffer from particle agglomeration or clumping at high current densities, which reduces their selectivity for CO.
To address this issue, the research team developed a silver-silica composite catalyst to maintain the silver catalyst’s performance. The pH is regulated by hydroxide ions (OH⁻) interacting with silica, which dissolve into a silicate form and precipitate under neutral conditions. This method addresses performance degradation at higher current densities without altering the catalyst's physical structure.
Unlike commercial silver catalysts, which lose selectivity at high current densities (around 60 % at 800 mA cm⁻²), the silver-silica composite catalyst maintained nearly 100 % selectivity even at a higher current density of 1 A cm⁻². Additionally, the catalyst significantly improved CO₂ conversion to CO by about 47 % at high current densities.
The silver-silica composite catalyst enhances CO₂ reduction performance and durability, improving the commercial potential of CCU technology for electrochemical CO₂ conversion. Its excellent CO selectivity and durability due to reversible cycling allow for long-term performance, increasing productivity and economic feasibility.
Looking ahead, the team plans to optimize manufacturing processes for high-efficiency catalysts and conduct long-term durability testing, with potential applications in industrial facilities such as power plants and petrochemical plants.
The research provides a significant direction in enhancing catalyst reversibility and environmental control strategies for electrochemical systems. It is expected to contribute to the future demonstration and commercialization of electrochemical systems.
Dr. Hyung-Suk Oh, Clean Energy Research Center, Korea Institute of Science and Technology
Journal Reference:
Lim, C. et. al. (2024) Breaking the current limitation of electrochemical CO2 reduction via a silica-hydroxide cycle. Energy & Environmental Science. doi.org/10.1039/D4EE00448E