Reviewed by Lexie CornerDec 19 2024
Researchers from the Institute of Science Tokyo, led by Assistant Professor Megumi Okazaki, have identified key factors affecting water-splitting efficiency in a study published in Chem Catalysis. The study examines the roles of pH levels, metal oxide (MOx) catalysts, and Ru(II) photosensitizers.
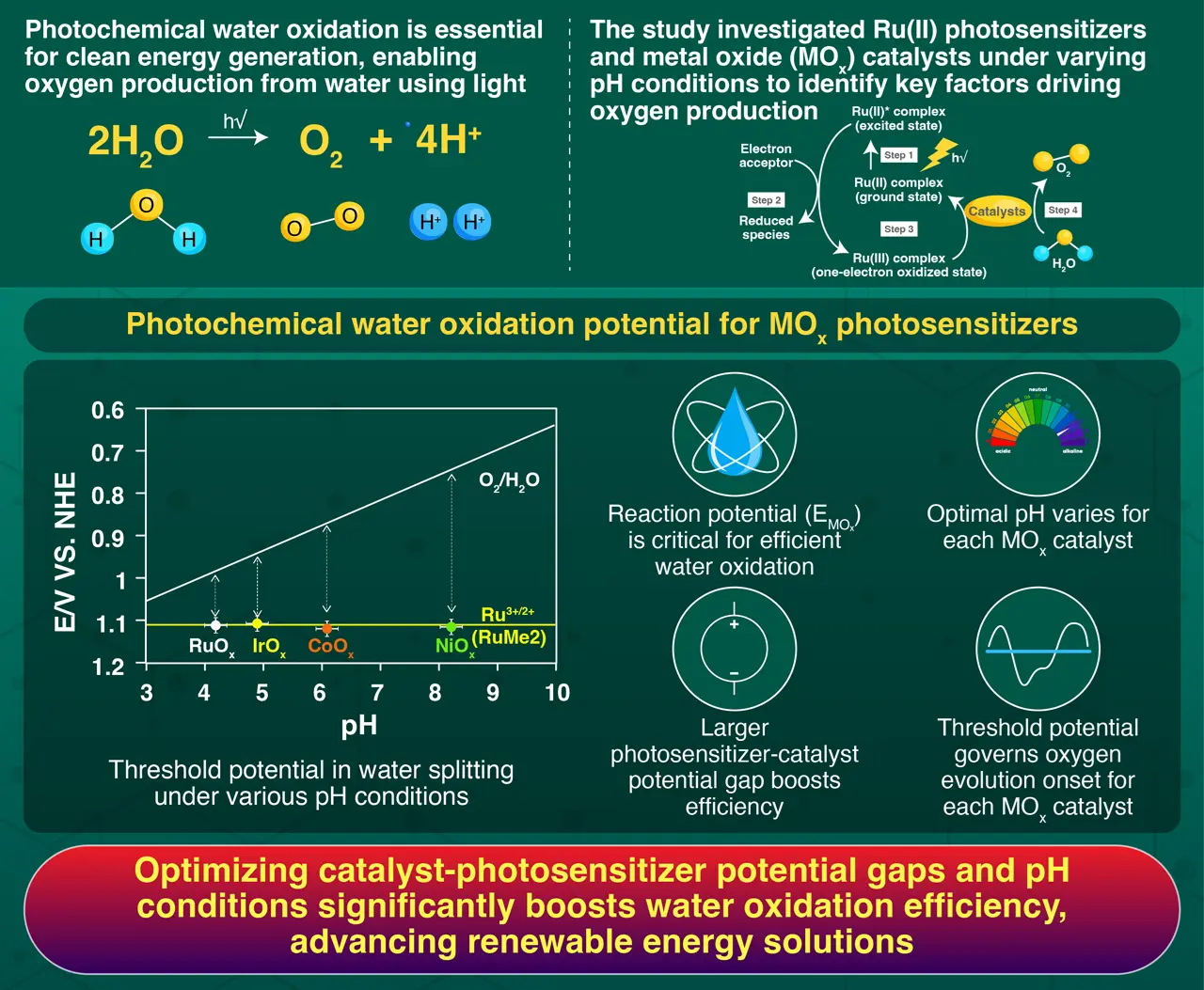 Discovery of the threshold potential that triggers photochemical water oxidation with Ru(II) photosensitizers and MOx catalysts. Image Credit: Okazaki et al. (2025) | Chem Catalysis | 10.1016/j.checat.2024.101167
Discovery of the threshold potential that triggers photochemical water oxidation with Ru(II) photosensitizers and MOx catalysts. Image Credit: Okazaki et al. (2025) | Chem Catalysis | 10.1016/j.checat.2024.101167
Water oxidation, which involves the production of oxygen, offers a promising pathway toward sustainable energy. This study investigates how water-splitting efficiency can be enhanced by adjusting pH levels, metal oxide catalysts, and Ru(II) photosensitizers.
To aid in the development of more efficient systems, researchers introduced a streamlined method for estimating catalyst performance. These findings provide valuable insights for advancing clean energy technologies and accelerating the transition to renewable energy sources.
As the global shift toward sustainable energy intensifies, the need for effective clean energy solutions has become increasingly urgent. Photochemical water oxidation—a process that uses light to split water molecules, releasing oxygen—represents a potential solution. While this technique shows significant promise, the precise role of catalysts in enhancing its efficiency remains unclear.
In this study, researchers evaluated the performance of Ru(II) photosensitizers with various MOx catalysts at different pH levels. Using a novel approach, they measured the reaction potential (EMOx) of the catalysts without requiring complex electrochemical setups. The data were analyzed to identify the thresholds for oxygen evolution and to assess how the potential gap between the catalyst and photosensitizer influences efficiency.
The study identified several factors that impact the effectiveness of water oxidation, providing critical information for improving this process and advancing clean energy solutions.
Reaction potential (EMOx) plays a critical role in the water oxidation process, directly visualizing the driving force towards water oxidation that have never measured by any apparatus under reaction condition.
Megumi Okazaki, Assistant Professor, Institute of Science Tokyo
The findings reveal that different MOx catalysts have unique onset pH conditions that determine whether water oxidation can occur. This highlights the importance of tailoring reaction conditions to the specific catalyst. The study also emphasized the significance of threshold potential—the point at which each catalyst begins producing oxygen and the reaction starts.
The research demonstrated that adjusting pH and reaction potential can significantly improve water oxidation efficiency. It provides a strategic framework for optimizing reaction conditions for individual catalysts.
“By developing a simplified method to estimate reaction potentials, we are making this research more accessible and cost-effective. This innovation could revolutionize the way we design and select catalysts, accelerating progress toward more efficient and sustainable energy solutions,” Okazaki added.
These results represent a step forward in improving clean energy production. By optimizing reaction conditions, scientists can enhance efficiency, reduce reliance on fossil fuels, and expand access to renewable energy technologies. The novel approach to estimating reaction potentials could reshape how researchers design and evaluate catalysts, driving further advancements in the field.
This study lays the foundation for more efficient water oxidation systems by exploring the interactions between catalysts, photosensitizers, and pH. It brings us closer to practical solutions for the energy challenge and holds the potential to transform sustainable energy production. Each step forward in renewable energy research brings us closer to a greener, more sustainable future.
Photoelectrochemical (PEC) Water Splitting for Hydrogen Production
Journal Reference:
Okazaki, M., et. al. (2024) Discovery of the threshold potential that triggers photochemical water oxidation with Ru(II) photosensitizers and MOx catalysts. Chem Catalysis. doi.org/10.1016/j.checat.2024.101167