Reviewed by Lexie CornerJan 6 2025
Researchers at Shandong University tackled the issues of low conductivity and iodine migration in zinc-iodine batteries by designing a novel cathode material. By embedding single-atom Zn catalysts with molybdenum carbide clusters in a hierarchical porous carbon framework, they improved iodine adsorption and catalytic activity. The study was published in Science Bulletin.
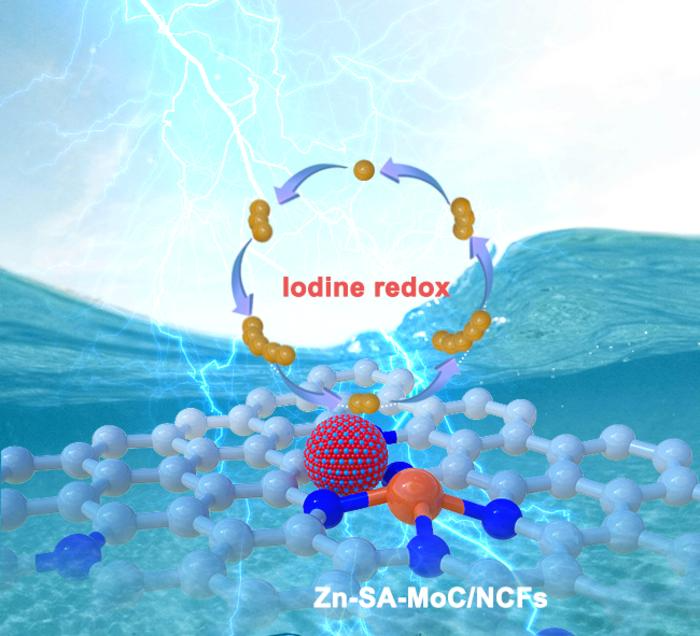 Molybdenum carbide (MoC) nanoclusters embedded in porous nitrogen-doped carbon fibers with atomic Zn-N4 sites exhibit a physicochemical confinement effect on iodine species and enhance electron/ion transfer efficiency, facilitating the reversible redox conversion without polyiodide shuttle effects. Image Credit: Science China Press
Molybdenum carbide (MoC) nanoclusters embedded in porous nitrogen-doped carbon fibers with atomic Zn-N4 sites exhibit a physicochemical confinement effect on iodine species and enhance electron/ion transfer efficiency, facilitating the reversible redox conversion without polyiodide shuttle effects. Image Credit: Science China Press
Aqueous zinc-ion batteries (ZIBs) have gained attention for their safety, abundance, and environmental benefits. Iodine, found in seawater (55 μg L−1), is a promising material for zinc-iodine batteries due to its high theoretical capacity (211 mAh g−1) and suitable redox potential (0.54 V).
However, iodine’s low electrical conductivity limits the redox conversion needed for effective energy storage. Additionally, soluble polyiodides produced during the process can migrate to the zinc anode, causing corrosion and capacity loss.
To address these challenges, the research team used a coprecipitation method to encapsulate molybdate ions within zeolitic imidazolate framework-8 (ZIF-8). This was followed by electrospinning and calcination, resulting in free-standing porous carbon fibers embedded with zinc single-atom sites and molybdenum carbide clusters (Zn-SA-MoC/NCFs).
The integration of molybdenum carbides with single-atom catalysts improves iodine species adsorption and optimizes catalytic activity through charge redistribution. The hierarchical porous carbon framework further facilitates mass transfer. As a result, the Zn-I2 batteries achieved a high specific capacity of 230.6 mAh g−1 at a current density of 0.5 C (1 C = 0.211 mA cm−2) and retained 90 % capacity after 20,000 cycles.
The study highlights the modification of electronic structures between hosts and iodine species as a key factor for enhancing Zn-I2 battery performance. It also demonstrates how Zn-N4 sites enhance the electrocatalytic activity of MoC clusters for iodine redox reactions. This electronic structure regulation approach provides valuable insights for developing advanced iodine catalysts and optimizing battery performance.
Journal Reference:
Chen, S., et al. (2024) Exploring interfacial electrocatalysis for iodine redox conversion in zinc-iodine battery. Science Bulletin. doi.org/10.1016/j.scib.2024.11.042