A research team from the Chinese Academy of Sciences’ (CAS) Dalian Institute of Chemical Physics (DICP), led by Prof. Dehui Deng, Assoc. Prof. Xiaoju Cui, and Prof. Liang Yu, used a nano-heterostructure catalyst to successfully accomplish the extremely efficient photo-driven carbonylation of CH4 with CO and O2 to CH3COOH in a study published in Nature Communications.
 Schematic illustration of the photo-driven CH4 carbonylation with CO and O2 to CH3COOH over the RhZn-MoS2/TiO2 and the comparison of catalytic activity for different catalysts. Image Credit: LI Yanan and LIU Huan.
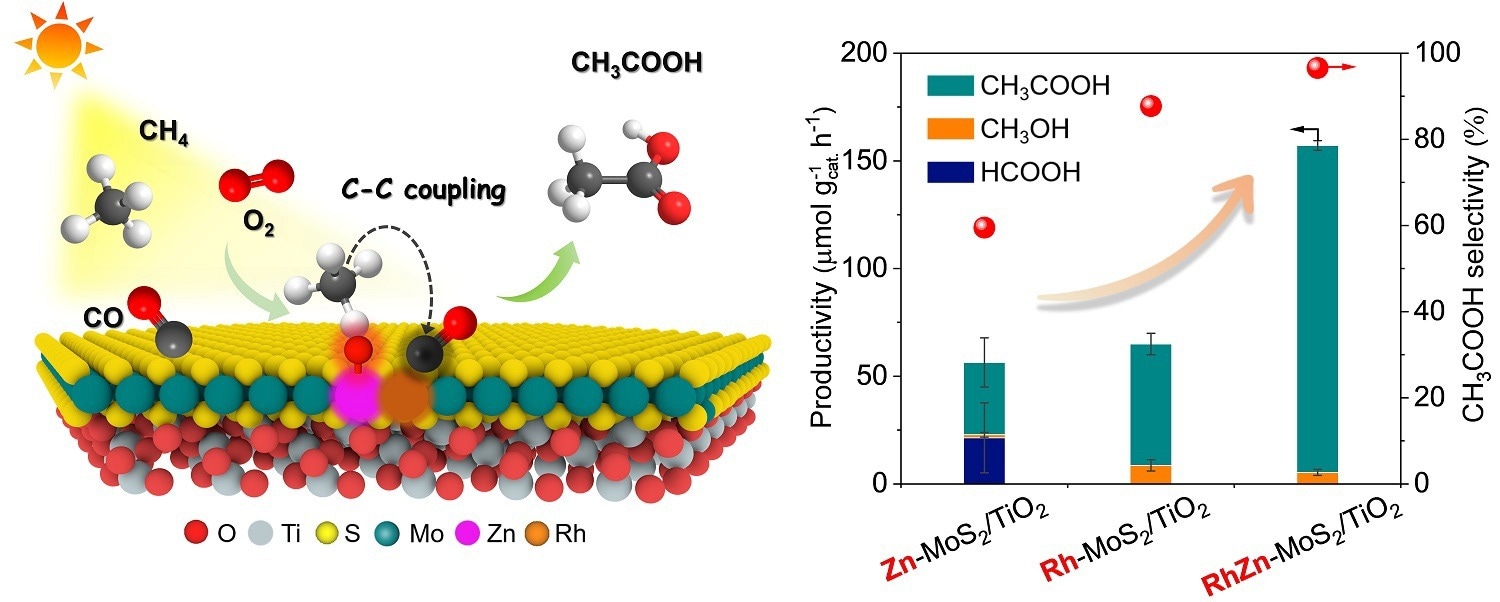
Schematic illustration of the photo-driven CH4 carbonylation with CO and O2 to CH3COOH over the RhZn-MoS2/TiO2 and the comparison of catalytic activity for different catalysts. Image Credit: LI Yanan and LIU Huan.
Under moderate circumstances, directly converting methane (CH4) to high-value-added multi-carbon (C2+) oxygenates, such as acetic acid (CH3COOH), represents a viable approach for upgrading natural gas to transportable liquid chemicals.
Under moderate circumstances, oxidative carbonylation of CH4 with carbonic oxide (CO) and oxygen (O2) to CH3COOH is a desirable and ecologically acceptable method of using CH4. However, this mechanism requires complicated reactions, such as O2 activation, efficient CH4 activation, and controlled C-C coupling. As a result, converting CH4, CO, and O2 to CH3COOH with high catalytic activity and selectivity in mild circumstances is a significant difficulty.
This catalyst has Rh-Zn atomic-pair dual sites inside a MoS2 lattice and TiO2 nanoparticles. This catalyst achieves CH3COOH productivity of 152 μmol gcat–1 h–1, turnover frequency of 62 h–1, and good selectivity of 96.5%.
With a high selectivity of 96.5%, this novel catalyst allows for a CH3COOH productivity of 152 μmol gcat.-1 h-1 and a turnover frequency of 62 h-1.
Furthermore, the researchers discovered that the active OH species generated by O2 photoreduction at the Zn site via proton-coupled electron transfer increase CH4 dissociation into CH3 species. These CH3 species subsequently readily pair with adsorbed CO on the neighboring Rh site, resulting in highly selective CH3COOH production.
The Rh-Zn atomic-pair dual sites provide distinct catalytic sites for C-H activation and C-C coupling, resulting in a synergistic effect that overcomes the traditional trade-off between activity and selectivity in CH4 carbonylation.
Our study opens a new horizon to design efficient catalyst and provides a new pathway for photo-driven CH4 carbonylation to CH3COOH.
Dehui Deng, Professor, Dalian Institute of Chemical Physics, Chinese Academy of Sciences
Journal Reference:
Li, Y., et al. (2025) MoS2-confined Rh-Zn atomic pair boosts photo-driven methane carbonylation to acetic acid. Nature Communications. doi.org/10.1038/s41467-024-54061-z