Reviewed by Lexie CornerFeb 11 2025
A study published in ACS Materials Letters by researchers at the U.S. Department of Energy's (DOE) Argonne National Laboratory examined solid electrolytes for all-solid-state batteries. The findings contribute to the development of safer and more energy-efficient battery technologies.
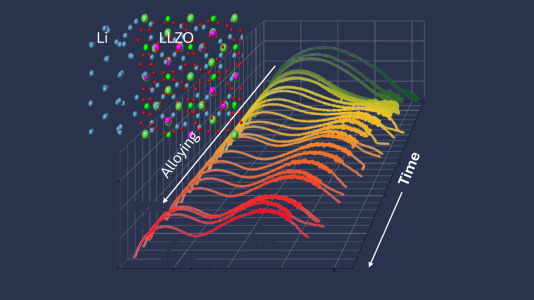 At the interface of lithium lanthanum zirconium garnet and lithium metal, potential dopant positions are shown as pink spheres. The colorful waves reveal gallium reduction and alloy formation after lithium deposition. Image Credit: Matt Klenk, Sanja Tepavcevic and Peter Zapol/Argonne National Laboratory.
At the interface of lithium lanthanum zirconium garnet and lithium metal, potential dopant positions are shown as pink spheres. The colorful waves reveal gallium reduction and alloy formation after lithium deposition. Image Credit: Matt Klenk, Sanja Tepavcevic and Peter Zapol/Argonne National Laboratory.
Lithium-ion batteries power a range of devices, including cell phones, laptops, and electric vehicles. Given their widespread use, researchers continue to explore ways to enhance battery safety and efficiency.
Electrolytes act as membranes that facilitate lithium-ion transport between a battery’s positive and negative electrodes. Unlike conventional lithium-ion batteries, which use liquid electrolytes, all-solid-state batteries employ solid electrolytes. These materials offer higher energy density, longer lifespan, and improved safety, as they are neither volatile nor flammable.
Solid electrolytes are also less reactive with lithium metal, making them more suitable for lithium metal electrodes compared to liquid electrolytes. Lithium metal has a higher energy density than graphite, a conventional electrode material, because all its atoms participate in charge and discharge cycles.
Lithium lanthanum zirconium garnet (LLZO) is a promising solid electrolyte due to its stability, durability, and high ionic conductivity, which enables efficient lithium-ion transport between electrodes. Researchers have explored doping LLZO with elements such as aluminum or gallium to enhance its conductivity. Doping involves introducing small amounts of another element to modify the material’s properties.
Doping with aluminum or gallium helps LLZO retain its most symmetric structure and introduces vacant sites that facilitate lithium-ion movement, improving conductivity. However, doping can also increase LLZO’s reactivity with lithium metal, which can reduce battery cycle life.
To understand this tradeoff, researchers examined the interaction between doped LLZO and metallic lithium using computational and experimental methods. They found that gallium is more mobile and readily forms an alloy with lithium, leading to its depletion from LLZO. This depletion alters the lithium garnet’s structure and reduces its ionic conductivity. In contrast, aluminum-doped LLZO remains more stable.
Gallium-doped LLZO offers higher ionic conductivity than aluminum-doped LLZO, but its reactivity with lithium suggests that an interfacial layer is necessary to maintain conductivity while preventing degradation.
These findings provide insight into how different dopants influence LLZO’s performance and stability, informing the development of more reliable solid-state batteries.
It is important to know how a dopant will react with lithium. It is another requirement for good electrolytes, not just high conductivity.
Peter Zapol, Study Lead Researcher and Physicist, Argonne National Laboratory
Sanja Tepavcevic, an Argonne chemist and the study's lead experimentalist, said that if dopants are unstable, enhanced conductivity is insufficient.
If we can separate reactivity from conductivity, or if we can develop one material that has both high conductivity and stability, that’s basically what we are trying to show with this work.
Sanja Tepavcevic, Study Lead Experimentalist, Argonne National Laboratory
By integrating computational and experimental approaches, researchers measured key properties of doped materials while gaining atomic-level insights into the interactions between lithium metal and solid electrolytes.
Using density functional theory, a computational method for modeling atomic and electronic behavior in materials, they predicted dopant stability and its interactions with other components.
Few experimental techniques allow direct examination of the solid electrolyte-electrode interface, particularly during electrochemical reactions in battery operation. Tepavcevic noted that these interfaces are “buried” and not easily accessible with conventional experimental methods.
To analyze surface chemistry changes in LLZO, the researchers used X-ray photoelectron spectroscopy. Electrochemical impedance spectroscopy was used to study lithium-ion mobility within the electrolyte and at the electrolyte-electrode interface.
Neutron diffraction, another experimental technique, was used to determine atomic arrangements in the material. This method confirmed that gallium became less stable and more reactive when interacting with lithium, whereas aluminum remained stable.
The study benefited from collaborations with institutions such as the University of California, Santa Barbara, which provided high-quality LLZO samples. Neutron diffraction experiments were conducted at the Nuclear Physics Institute of the Czech Academy of Sciences in the Czech Republic and the Heinz Maier-Leibnitz Zentrum in Germany.
Zapol added, “The role of the US-German collaboration was absolutely critical for this work. Looking ahead, these findings open new avenues in the international pursuit of safer, more efficient solid-state batteries.”
The study was supported by the US-German Cooperation on Energy Storage, established by the DOE's Office of Energy Efficiency and Renewable Energy for the Vehicle Technologies Office to facilitate collaborative research on lithium batteries.
Argonne contributors include Yisi Zhu, Justin Connell, Zachary Hood, Michael Counihan, and Matthew Klenk, along with Tepavcevic and Zapol. Additional contributions were made by Jeff Sakamoto from the University of California, Santa Barbara; Charles Hervoches from the Nuclear Physics Institute of the Czech Academy of Sciences; and Neelima Paul and Ralph Gilles from the Heinz Maier-Leibnitz Zentrum.
Journal Reference:
Klenk, M. et. al. (2025) Comparative Analysis of Reactivity of Al and Ga Doped Garnet Solid State Electrolyte at the Interface with Li Metal. ACS Materials Letters. doi.org/10.1021/acsmaterialslett.4c01237