Researchers from the Institute of Science Tokyo opened the door for sustainable chemical innovation by revealing Ba-Si orthosilicate oxynitride-hydride as a catalyst that is free of transition metals. The study was published in Nature Chemistry.
 Anion vacancies activate N2 to ammonia on Ba-Si orthosilicate oxynitride-hydride. Image Credit: Zhang et al. (2025) | Nature Chemistry
Anion vacancies activate N2 to ammonia on Ba-Si orthosilicate oxynitride-hydride. Image Credit: Zhang et al. (2025) | Nature Chemistry
The study aims to develop more efficient and sustainable ammonia synthesis methods.
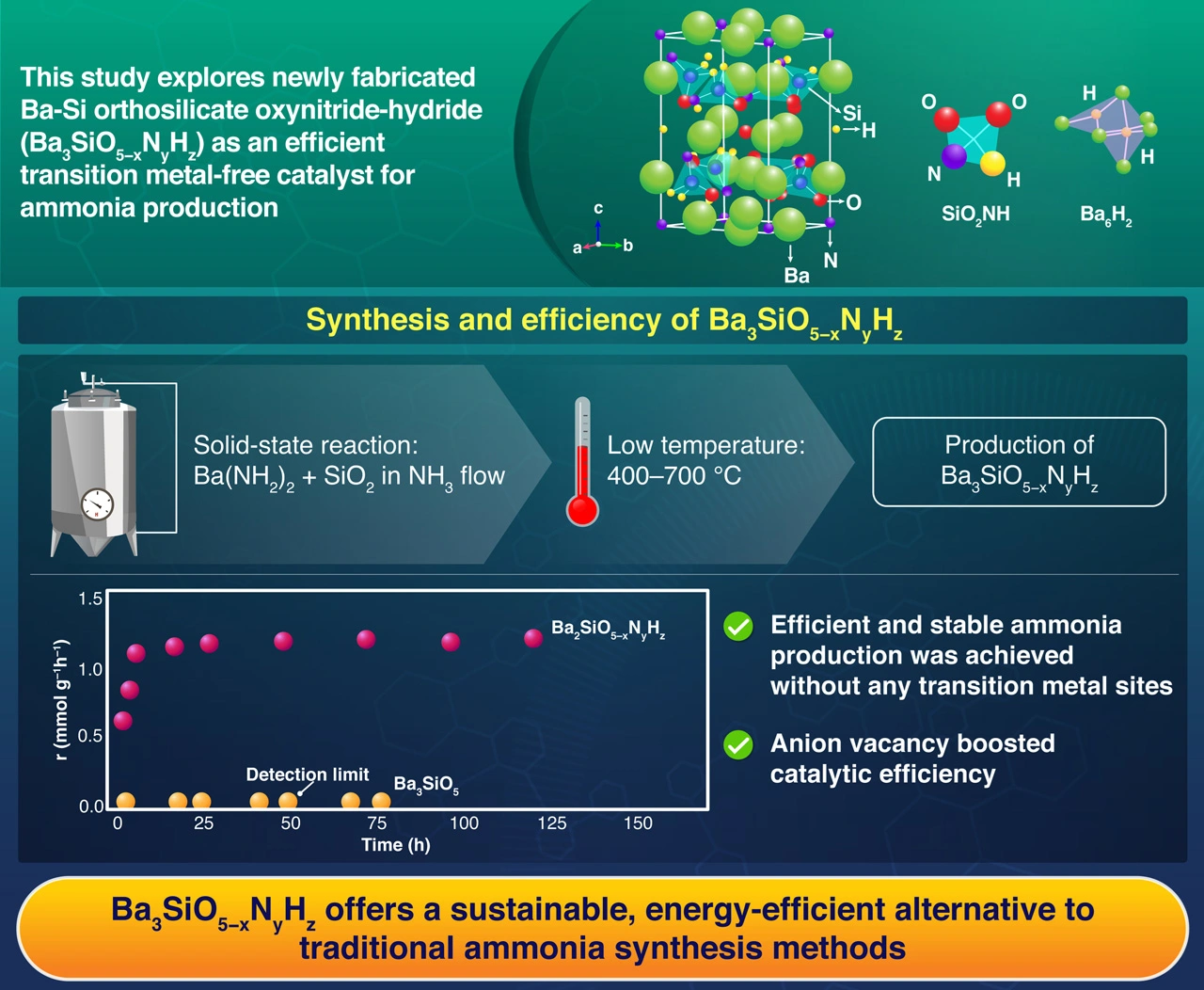
The study investigates Ba-Si orthosilicate oxynitride-hydride (Ba3SiO5−xNyHz) as a viable substitute for conventional transition metal-based systems in the ammonia synthesis process. These compounds, which were synthesized using low-temperature solid-state reactions and supplemented with ruthenium nanoparticles, showed enhanced catalytic performance in milder conditions, offering a more energy-efficient method of producing ammonia.
This strategy also tackles the environmental issues with traditional techniques, indicating a move toward more environmentally friendly industrial processes for ammonia production.
The need for effective substitutes in all sectors of the economy is only increasing as the world becomes more sustainable. Ammonia is a vital component of fertilizers, explosives, and many other products, and it is mainly produced using the energy-intensive Haber-Bosch process. This process requires extremely high pressures and temperatures, which raises carbon dioxide emissions worldwide.
These severe circumstances are necessary for the reaction to be fueled by traditional catalysts like iron and ruthenium. A new study led by Professor Masaaki Kitano of Tohoku University, Japan, the National Institute for Materials Science, and the Institute of Science Tokyo investigates the Ba3SiO5−xNyHz catalyst as a sustainable substitute for conventional catalysts that could transform ammonia synthesis.
Active sites are voids, particularly anion vacancies in the three-dimensional structure of catalysts. These active sites actively participate in the catalysis process. However, without transition metal sites, anion vacancies are ineffective on their own. This restriction prompted scientists to create a catalyst devoid of transition metals.
Kitano explained, “We have focused on tribarium silicate (Ba3SiO5) for the synthesis of our novel catalyst due to its unique crystal structure and chemical properties, offering the potential to lower energy requirements and reduce operating conditions,” describing the initial step in their research.
The research team created and evaluated several mixed-anion materials to address the energy and environmental issues raised by traditional synthesis techniques.
The research went through several phases. First, the researchers used a low-temperature (400–700 °C) solid-state reaction of barium amide with silicon dioxide to create a new Ba-Si oxynitride-hydride, Ba3SiO5−xNyHz. The final chemical composition was Ba3SiO2.87N0.80H1.86.
Compared to the synthesis temperatures (1100–1400 °C) of traditional silicate materials like Ba3SiO5, Ba3Si6O9N4 and BaSi2O2N2, this temperature is substantially lower. Even without any transition metal sites, the synthesized Ba3SiO5−xNyHz showed remarkable stability as a catalyst for ammonia synthesis. Compared to the traditional ruthenium-loaded MgO catalyst, it demonstrated greater activity and lower activation energy.
However, Ba3SiO5, Ba3Si6O9N4, and BaSi2O2N2 did not show any catalytic activity. Advanced instrumentation techniques were used to analyze the structural properties and test the ammonia synthesis activity of the Ba3SiO5−xNyHz catalyst under different pressures and temperatures.
Ruthenium nanoparticles were added to enhance performance even more. Using ruthenium nanoparticles, the researchers discovered that Ba3SiO5−xNyHz exhibited the highest catalytic activity.
The addition of ruthenium nanoparticles significantly boosted catalytic performance, enabling more efficient ammonia synthesis under milder conditions. However, the main active site is not ruthenium nanoparticles but the anion vacancy sites on Ba3SiO5−xNyHz, which reduces the apparent energy requirement for ammonia synthesis than conventional catalysts. We also discovered that the anion vacancy-mediated mechanism played a key role in facilitating nitrogen activation, without relying on transition metals.
Masaaki Kitano, Professor, Tohoku University
These results point to a more energy-efficient and sustainable route for ammonia synthesis.
The transition metal-free pathway reduces emissions and resource dependence, supporting sustainability, while lower temperatures and pressures improve efficiency, according to the study. Additionally, Ba3SiO5−xNyHz catalysts' robust performance and scalable synthesis make them attractive options for industrial adoption, providing a more environmentally friendly method of producing ammonia on a large scale. These results pave the way for future studies on transition metal-free catalysis for other important processes.
This study addresses a significant industrial chemistry challenge and is a major step toward sustainable ammonia synthesis. By showcasing the capabilities of the Ba3SiO5−xNyHz catalyst, the scientists have established the groundwork for a more environmentally friendly and effective method of creating ammonia, a necessary chemical. As the demand for ammonia rises globally, innovations like this one will fuel a more sustainable future.
Journal Reference:
Zhang, Z., et al. (2025) Anion vacancies activate N2 to ammonia on Ba–Si orthosilicate oxynitride-hydride. Nature Chemistry. doi.org/10.1038/s41557-025-01737-8