For the first time, researchers at the University of Minnesota Twin Cities have discovered a method to selectively burn a single molecule within a mixture of hydrocarbons—compounds composed of hydrogen and carbon atoms.
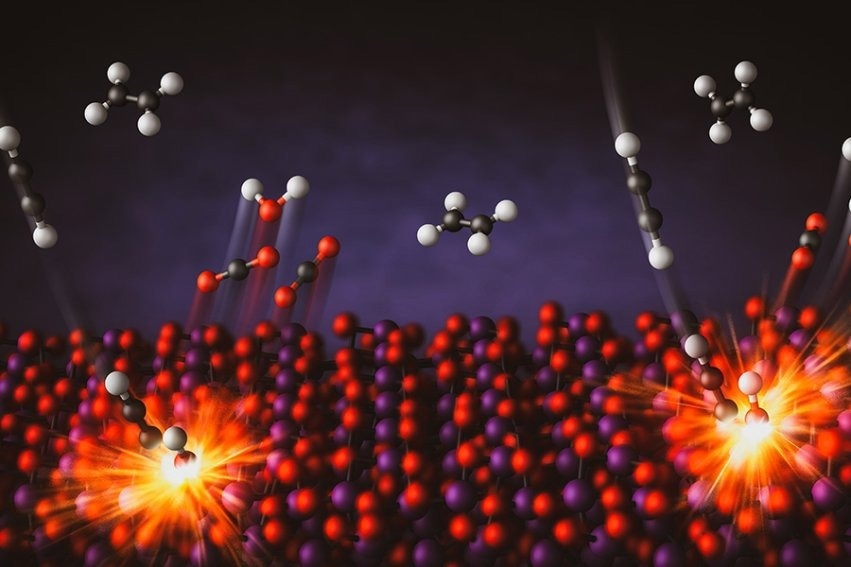
This illustration depicts the combustion of small amounts of acetylene in mixtures with ethylene. Image Credit: Greg Stewart/SLAC National Accelerator Laboratory
This breakthrough could significantly aid in pollutant removal and enhance efficiency across various industrial processes, including the production of fuels, medications, fertilizers, and plastics.
The study is published in Science, a leading international, peer-reviewed scientific journal.
Using a bismuth oxide catalyst—a substance that accelerates chemical reactions—the researchers demonstrated the ability to selectively combust one molecule in a mixture of combustibles. Their findings show that even small amounts of acetylene can be effectively burned in mixtures containing ethylene. Removing acetylene is crucial for preventing the poisoning of polymerization catalysts, a key step in the production of polyethylene plastics—a market that exceeds 120 million metric tons annually.
No one else has shown that you could combust one hydrocarbon present in low concentrations, in mixtures with others.
Aditya Bhan, Study Lead Investigator, Distinguished McKnight University Professor, Department of Chemical Engineering and Materials Science, University of Minnesota
Traditionally, combustion processes burn entire hydrocarbon fuel mixtures at high temperatures to generate heat. However, the use of a catalyst enabled researchers to selectively target one molecule without affecting others. What makes the bismuth oxide catalyst particularly unique is its ability to supply its own oxygen during combustion, eliminating the need for an external oxygen source through a process known as chemical looping.
We were able to take oxygen out of the catalyst and put it back in multiple times, where the catalyst changes slightly, but its reactivity is not impacted. Operating in this chemical looping mode avoids flammability concerns.
Matthew Jacob, Study First Author and PhD Candidate, University of Minnesota
Eliminating small concentrations of contaminants is typically challenging and energy-intensive. This new approach presents a more energy-efficient alternative.
You want to do this process selectively. Removing acetylene and other trace hydrocarbon contaminants in this manner could be more energy efficient. You just want to be able to go into a gas mixture to remove some molecules without touching the rest of the molecules.
Matthew Neurock, Study Senior Co-Author and Professor, Department of Chemical Engineering and Materials Science, University of Minnesota
The researchers emphasized that the long-term impact of this discovery could be substantial, as catalysts play a critical role in producing everyday materials, from fuels and pharmaceuticals to fertilizers and plastics. Gaining insight into how molecules combust—or resist combustion—on catalyst surfaces could lead to more efficient production processes for fuels and plastics.
“If we can understand how a catalyst works, at a molecular atomic level, we can adapt it to any particular reaction,” This can help us understand how catalysts, that produce fuels and chemicals needed in modern living, react to their environment,” added Simon Bare, a Distinguished Scientist at the SLAC National Accelerator Laboratory at Stanford University, and co-author of the study.
Journal Reference:
Jacob, M. et. al. (2025) Selective chemical looping combustion of acetylene in ethylene-rich streams. Science. doi.org/10.1126/science.ads3181