Scientists at Stanford Synchrotron Radiation Lightsource (SSRL) have discovered how platinum electrodes dissolve, which could lead to advancements in renewable energy. The study was published in Nature Materials.
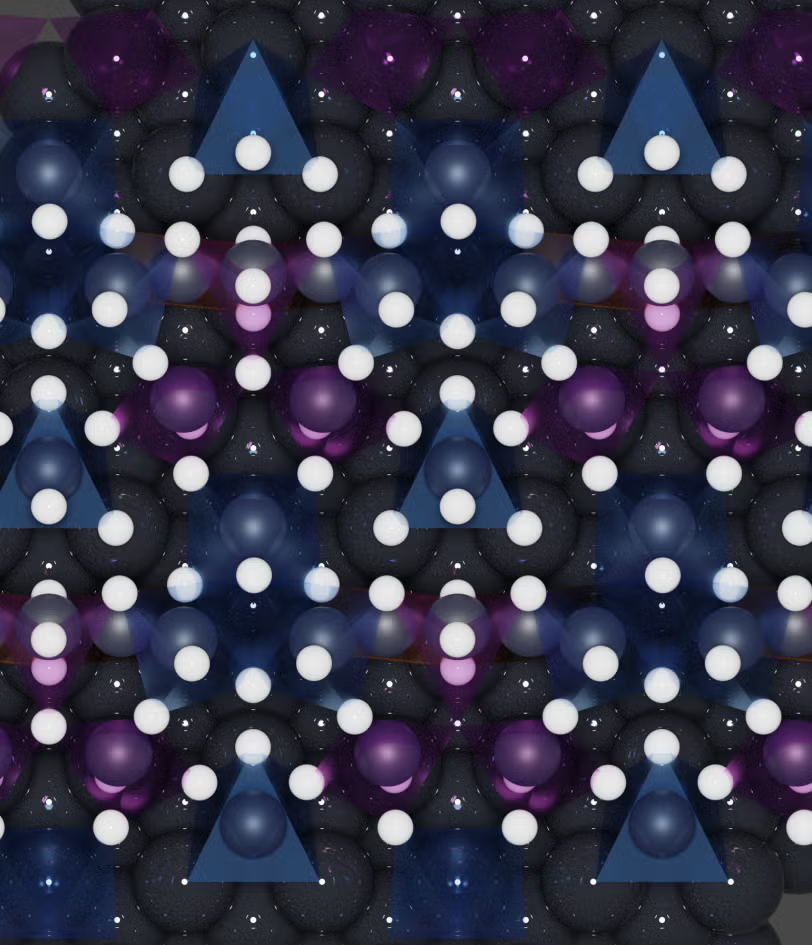 A top-view visualization of a platinum surface during cathode corrosion. Platinum and hydrogen atoms are represented in black and white, respectively. Blue and purple triangles indicate where hydrogen atoms have been bound to platinum atoms. Image Credit: Selwyn Hanselman/Leiden University.
A top-view visualization of a platinum surface during cathode corrosion. Platinum and hydrogen atoms are represented in black and white, respectively. Blue and purple triangles indicate where hydrogen atoms have been bound to platinum atoms. Image Credit: Selwyn Hanselman/Leiden University.
For almost 20 years, scientists have sought to unravel the mystery of how negatively polarized platinum electrodes corrode—a challenge that affects water electrolyzers, a key hydrogen production technology, as well as electrochemical sensors that rely on platinum electrodes.
Researchers from Leiden University and the Department of Energy's SLAC National Accelerator Laboratory have now closely collaborated to identify the culprit, which could lead to more dependable electrochemical sensors and less expensive hydrogen energy production.
Negatively polarized platinum electrodes immersed in an electrolyte, which is essentially saltwater, are a common component of electrolyzers and many other electrochemical devices.
That option is costly but durable and generally stable.
But being quite stable doesn't mean it doesn't degrade.
Dimosthenis Sokaras, Senior Scientist and Principal Investigator, Stanford Synchrotron Radiation Lightsource (SSRL)
Dimosthenis Sokaras is the principal investigator of the SLAC team.
Being negatively polarized prevents corrosion in most metals. However, platinum electrodes can degrade quickly under these circumstances, a peculiarity that has baffled scientists.
If you take a piece of platinum and you apply a very negative potential, you can dissolve your platinum in a matter of minutes.
Marc Koper, Professor and Principal Investigator, Department of Catalysis and Surface Chemistry, Leiden University
Two leading theories have been proposed to explain this process. Some researchers believed that sodium ions from the electrolyte solution were responsible. The theory suggested that these ions pushed into the platinum’s atomic lattice, forming platinides—platinum atoms bonded with positively charged sodium ions—that gradually peel away. Others proposed a similar mechanism, but instead blamed the production of platinum hydrides on the cooperation of sodium and hydrogen ions, or protons.
The research team was aware that they would have to observe platinum in some way because it was corroding in an electrolyte and producing a lot of hydrogen. To accomplish this, the team looked to SSRL, where scientists have created high-energy-resolution X-ray spectroscopy methods that can pass through the electrolyte and eliminate other influences, enabling them to concentrate on minute variations in the platinum electrode in operando, or while it is operating.
High-energy-resolution X-ray absorption spectroscopy, for us, was the only technique we could come up with that could sort of deal with the experimental conditions.
Thom Hersbach, Scientist, SLAC
The team developed a special “flow cell” that could clear hydrogen bubbles that form during the electrode’s operation and interfere with the X-ray experiment.
By recording X-ray spectra from the surface of the negatively polarized electrode, the team made the first-ever observations of platinum actively corroding.
Before experimenting, the researchers suspected that hydrides caused the corrosion, but it took them years to analyze the data and validate the theory.
“It just took loads and loads of different iterations of trying to figure out 'how do we accurately capture what's going on?” said Hersbach.
The researchers simulated the spectra they anticipated seeing from each structure under the SSRL X-ray beam using computer models of platinides and platinum hydrides. It was determined that only platinum hydride could have produced their results by comparing the many simulated spectra with the outcomes of their experiment.
“By advancing the frontiers of X-ray science, SSRL has developed operando methods that, combined with modern supercomputing, now allow us to tackle decades-old scientific questions,” said Sokaras.
Solutions for platinum corrosion in electrolyzers and numerous other electrochemical devices can now be developed.
Koper said, the project “demonstrates how important it is in science to put a lot of expertise together.”
Journal Reference:
Kroll, T., et al. (2025) Platinum hydride formation during cathodic corrosion in aqueous solutions. Nature Materials. doi.org/10.1038/s41563-024-02080-y