Researchers at the Indian Institute of Science have developed an innovative onsite production method for H₂O₂, which can also break down industrial pollutants such as toxic dyes. The scientists utilized a zinc-air battery in which oxygen reduction generates H2O2. The study was published in Small Methods.
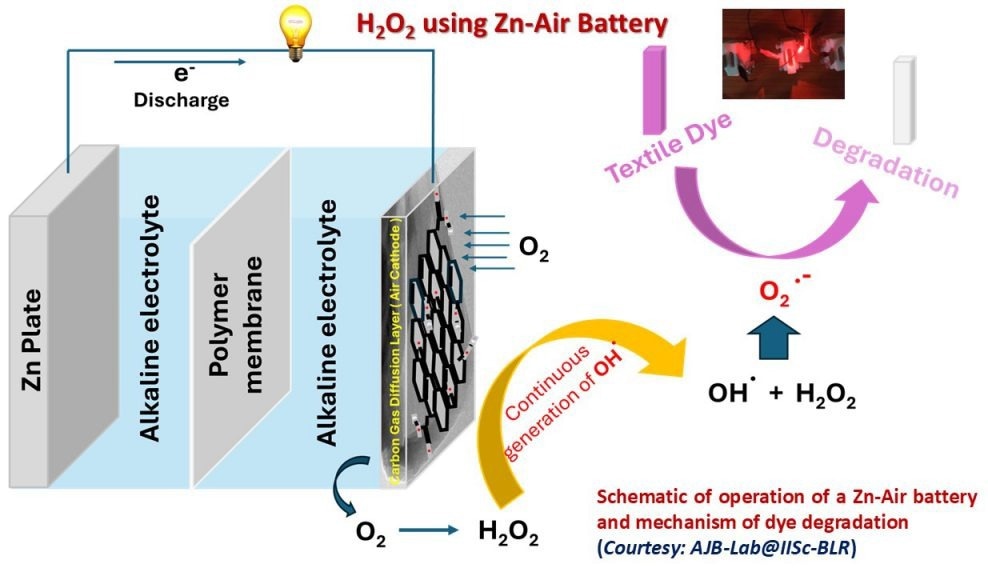 Schematic of operation of Zn-Air battery and dye degradation. Image Credit: AJB lab, IISc
Schematic of operation of Zn-Air battery and dye degradation. Image Credit: AJB lab, IISc
While hydrogen peroxide (H2O2) has numerous applications, its industrial production is costly and energy-intensive due to rare metal catalysts.
Zinc is an abundant and historically-used element … it is very cheap and abundant in India.
Aninda J Bhattacharyya, Professor and Study Corresponding Author, Solid State and Structural Chemistry Unit (SSCU), Indian Institute of Science
Aninda J Bhattacharyya is also associated with the Interdisciplinary Centre for Energy Research (ICER).
In a metal-air battery, zinc is the negative electrode, while air is the positive electrode. During discharge, the battery generates energy by reducing oxygen from the air at the cathode, producing hydrogen peroxide (H2O2).
Oxygen's electrochemical reduction can occur via two distinct pathways, one of which leads to the formation of hydrogen peroxide (H2O2).
The strategy here is to control the extent of the oxygen reduction reaction. If you don’t control it at some level, it will just go and form water.
Aninda J Bhattacharyya, Professor and Study Corresponding Author, Solid State and Structural Chemistry Unit (SSCU), Indian Institute of Science
Specific catalysts enable this level of control.
We are using a metal-free catalyst based on carbon.
Asutosh Behera, Study First Author and Ph.D Student, Solid State and Structural Chemistry Unit (SSCU), Indian Institute of Science
While cost-effective catalysts typically favor water production over hydrogen peroxide (H2O2), adding specific chemical modifications, such as oxygen functional groups, can redirect the reaction to selectively produce H2O2.
Bhattacharyya highlights the novelty of directly producing H2O2 using a battery.
“You don’t have to do other things. You have a battery, and you run it. We have curtailed the voltage such that it is only producing H2O2,” explained Bhattacharyya.
Batteries offer the advantage of simultaneous energy production and storage alongside chemical reactions. “What we are doing is that along with producing H2O2, we are storing energy because it takes place inside the cell,” Bhattacharyya notes.
To detect the colorless H2O2, a dye—a common toxic pollutant from the textile industry—is introduced. The H2O2 then reacts with and degrades the dye, causing a color change.
“The H2O2 generated will further decompose into various radicals (such as hydroxide and superoxide) – highly raw, reactive organic species – that will eventually degrade the textile dye,” explained Behera.
This degradation helps increase the efficiency of H2O2 production and eliminate the toxic dye.
“There are some fundamental challenges which must be overcome,” noted Bhattacharyya.
Metal-air batteries, with their solid, liquid, and gaseous phases, present handling complexities compared to typical two-phase batteries. However, researchers are optimistic about this approach's scalability and potential for applications like remote electricity generation.
“This method is very sustainable, low-cost, and highly energy-efficient,” concludes Bhattacharyya.
Journal Reference:
Behera, A. and Bhattacharyya, A. J., (2025) Employing a Zn-air/Photo-Electrochemical Cell for In Situ Generation of H2O2 for Onsite Control of Pollutants. Small Methods. doi.org/10.1002/smtd.202401539