Researchers at the University of Chicago Pritzker School of Molecular Engineering (UChicago PME) and the Tandon School of Engineering at New York University have made significant progress in understanding how water moves charged ions across a critical component in clean energy technologies like fuel cells and redox flow batteries.
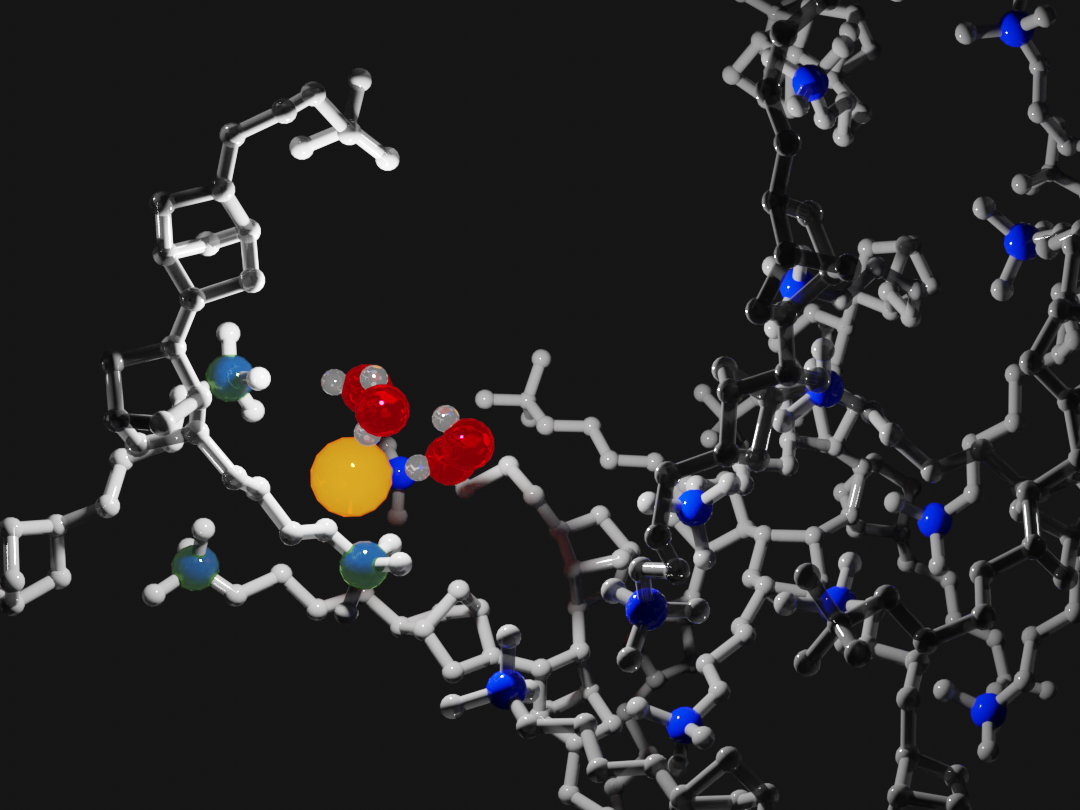 Combining simulations and experimental data let PME researchers build a picture of how negatively charged anions (yellow) and positively charged cations (blue) interact with water molecules (red and white) and the polymer backbone of anion exchange membranes (gray). Image Credit: Ge Sun
Combining simulations and experimental data let PME researchers build a picture of how negatively charged anions (yellow) and positively charged cations (blue) interact with water molecules (red and white) and the polymer backbone of anion exchange membranes (gray). Image Credit: Ge Sun
Until now, scientists believed that anion exchange membranes (AEMs) required high levels of free-flowing water for efficient ion transport. However, this new study challenges that assumption, showing that fast ion transport does not necessarily depend on excess free water.
Instead, researchers found that AEMs can be optimized by maintaining just enough water to form well-connected networks of water molecules within the membrane. This structure also ensures a dynamic shell of water around the ions, enabling efficient movement without compromising membrane stability.
The findings were published in Nature Communications.
Our study challenges the long-held idea that fast ion transport in energy membranes requires excess free water – in reality, it’s the structure of the water network that matters, not just the amount.
Paul Nealey, Professor and Study Senior Author, Pritzker School of Molecular Engineering, University of Chicago
“This research provides us with a molecular-level blueprint for optimizing energy membranes, bringing us one step closer to more efficient fuel cells, better batteries, and more sustainable energy storage solutions,” said Juan de Pablo, Study Senior Author and Former Professor at UChicago PME and now at New York University.
Understanding Ion Flow
AEMs are thin, specialized materials embedded with positively charged molecules that attract and guide negatively charged ions (anions) while repelling positively charged ions (cations). These membranes play a key role in electrochemical devices, where charge differences help drive reactions, such as generating electricity in fuel cells or splitting water to produce clean hydrogen in electrolyzers.
The efficiency of AEMs depends on how easily ions move through them. Scientists have long known that water plays a role in ion transport, but maintaining high levels of free-flowing water can limit device performance in low-humidity environments. It can also cause AEMs to swell, stretch, and degrade over time.
In this study, researchers combined experimental data on AEM efficiency with computer simulations to better understand the role of water. Using advanced two-dimensional infrared spectroscopy (2D IR), they were able to capture ultrafast water dynamics at the molecular level.
By integrating these approaches, we can precisely model what happens to the water molecules around AEMs on a timescale of picoseconds.
Ge Sun, Graduate Student and Study Co-First Author, Pritzker School of Molecular Engineering, University of Chicago
Their findings revealed that water molecules absorbed into an AEM form a network of hydrogen bonds within its structure. Rather than relying on excess free water, this structured network and the water shells surrounding ions enable efficient ion movement. When water levels are too low, more energy is needed to move ions across the membrane. However, as the hydrogen bond network becomes more structured, ion transport becomes significantly easier.
“We observed that even without high levels of water, we see a boost to ionic conductivity and ion transport across the membrane. This happens because the water network is well-formed, and water molecules in the second layer can quickly adjust their orientation,” said Sun.
Designing More Efficient Membranes
In the past, engineers designing AEMs often erred on the side of using more water than necessary. This study suggests a better approach: optimizing water levels to maximize efficiency while maintaining structural integrity.
By uncovering how water molecules organize inside these membranes, we can design next-generation materials that work efficiently even in low-humidity environments, making clean energy technologies more practical and durable.
Shrayesh Patel, Associate Professor and Study Co-Author, Pritzker School of Molecular Engineering, University of Chicago
A key advance in this research was the use of 2D IR spectroscopy, paired with sophisticated molecular models, to reveal fine details of water dynamics in these systems. This combination of experimental data and simulations offers a powerful framework that could be applied to other scientific challenges involving molecular movement.
These findings open new doors for designing more resilient and efficient energy membranes, bringing clean energy technologies one step closer to real-world viability.
Journal Reference:
Wang, Z., et al. (2025) Water-mediated ion transport in an anion exchange membrane. Nature Communications. doi.org/10.1038/s41467-024-55621-z