The demand for direct methanol/formic acid-fuel cell technology development has been rising to achieve carbon neutrality. Methanol or formic acid is utilized as an e-fuel in a new technique to generate energy.
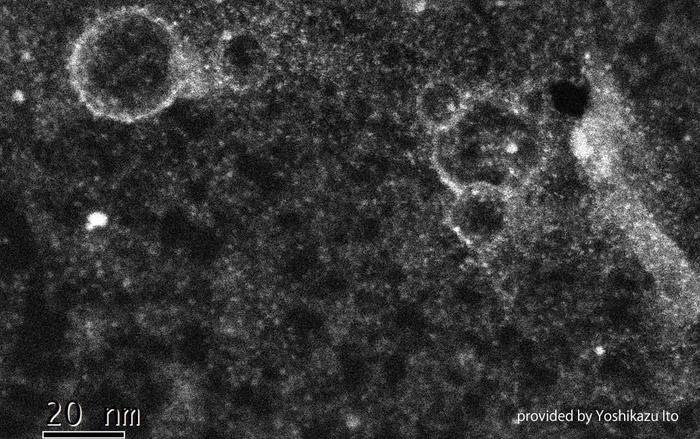 Morphological characterizations of sulfanilic-functionalized holey graphene; Transmission electron microscopy. Image Credit: University of Tsukuba
Morphological characterizations of sulfanilic-functionalized holey graphene; Transmission electron microscopy. Image Credit: University of Tsukuba
Electricity is generated via proton transfer in fuel cells; however, typical proton-exchange membranes suffer from the “crossover phenomenon,” in which fuel molecules are also moved between anodes and cathodes. The fuel molecules are oxidized, and the electrodes are turned off.
The researchers created a novel proton-exchange membrane out of graphene sheets with 5–10 nm-diameter holes that have been chemically modified with sulfanilic functional groups, resulting in sulfo groups around the holes. The graphene membrane efficiently suppresses the crossover phenomenon by limiting the entrance of the fuel molecules while preserving good proton conductivity for the first time, to the best of what is known, due to steric hindrance by the functional groups.
Until now, standard ways to prevent fuel-molecule movement required increasing the membrane thickness or sandwiching two-dimensional materials, which lowered proton conductivity. The researchers studied structures that restrict fuel molecule migration via electro-osmotic drag and steric hindrance in this study.
As a result, scientists discovered that the sulfanilic-functionalized graphene membrane could substantially minimize electrode deterioration when compared to commercially available Nafion membranes while still preserving the proton conductivity necessary for fuel cells.
Furthermore, adhering the graphene membrane to a standard proton-exchange membrane can decrease the crossover phenomenon. As a result, this study helps to progress the development of advanced fuel cells as a new option for hydrogen-type fuel cells.
JST-PRESTO (JPMJPR2115), the Mukai science and technology foundation, the JSPS Grant-in-Aid for Scientific Research on Innovative Areas “Discrete Geometric Analysis for Materials Design” (Grant Numbers JP20H04628, JP20H04639), JSPS KAKENHI (Grant Numbers JP21H02037, JP21KK0091, JP23K17661), and a cooperative program (Proposal No. 202111-CRKEQ-0001) of CRDAM-IMR, Tohoku University provided funding for the study.
Journal Reference:
Jeong, S., et al. (2023) Suppression of Methanol and Formate Crossover through Sulfanilic-functionalized Holey Graphene as Proton Exchange Membranes. Advanced Science. doi:10.1002/advs.202304082
Source: https://www.tsukuba.ac.jp/en/