Researchers from the University of Puerto Rico-Rio Piedras Campus have recently published a study in the journal Angewandte Chemie detailing the development of a groundbreaking ultralow-concentration electrolyte. This electrolyte has demonstrated outstanding performance in battery cells and shows promise in simplifying battery production and recycling processes.
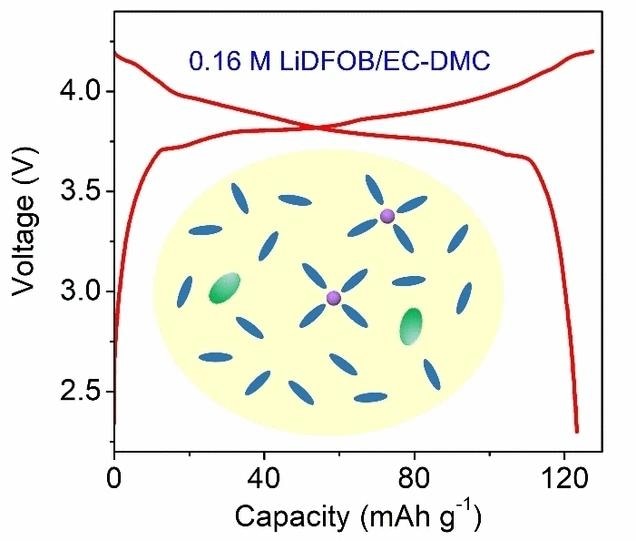
Image Credit: Angewandte Chemie
Lithium salts, while enhancing battery power, often contribute to high costs. However, an ultralow-concentration electrolyte utilizing the lithium salt LiDFOB may offer a more economical and sustainable alternative.
The study demonstrates that cells employing these electrolytes and conventional electrodes exhibit superior performance while also potentially streamlining battery production and recycling processes.
Lithium-ion batteries (LIBs) are ubiquitous power sources, fueling electric cars, powering tablets and smartphones, and storing energy in power plants. Comprising lithium cobalt oxide (LCO) cathodes, graphite anodes, and liquid electrolytes facilitating ion movement between cathode and anode, LIBs rely on these key components for their operation. Crucially, electrolytes regulate the formation of the interphase layer between electrodes, influencing battery cycling efficiency and other performance metrics.
Commercial electrolytes, however, continue to primarily rely on a system developed more than 30 years ago: carbonate solvent, or carboxylic acid esters containing 1.0–1.2 mol/L of lithium hexafluorophosphate (LiPF6).
In the past decade, there have been significant advancements in high-concentration electrolytes, exceeding 3 mol/L. These innovations have notably enhanced battery performance by promoting the formation of robust interphase layers dominated by inorganic compounds. However, despite these gains, such electrolytes face challenges, including high viscosity, limited wetting capability, and subpar conductivity. Moreover, their reliance on large quantities of lithium salts renders them prohibitively expensive, a crucial consideration for practical implementation.
In response to these cost concerns, researchers have turned their attention to the development of ultralow-concentration electrolytes with concentrations below 0.3 mol/L. While promising for cost reduction, these alternatives come with their own drawbacks. Namely, they tend to cause more solvent decomposition within the battery cell compared to the minimal salt anions present, resulting in the formation of a less stable interphase layer dominated by organic compounds.
The University of Puerto Rico-Rio Piedras Campus (USA) and Ningbo University (China) have collaborated to create an ultralow-concentration electrolyte called LiDFOB/EC-DMC that could be used in lithium-ion batteries. The team is led by Jinliang Yuan, Lan Xia, and Xianyong Wu.
LiDFOB, or lithium difluoro(oxalato)borate, serves as a cost-effective alternative to the commonly used LiPF6 additive. Paired with EC-DMC, an ethyl carbonate/dimethyl carbonate commercial carbonate solvent, this electrolyte achieves a record-breaking low salt content of just two weight percent (equivalent to 0.16 mol/L). Despite this minimal salt concentration, it exhibits a commendably high ionic conductivity of 4.6 mS/cm, rendering it viable for battery operation.
Furthermore, the unique properties of the DFOB– anions enable the formation of a robust, inorganic-dominated interphase layer on both LCO and graphite electrodes. This characteristic translates into outstanding cycling stability observed in both half and full cells, marking a significant advancement in battery technology.
While the LiPF6 in current use decomposes in the presence of moisture, releasing highly toxic and corrosive hydrogen fluoride gas (HF), LiDFOB is water- and air-stable. Instead of strict dry room conditions, LIBs with LiDFOB can be made under ambient conditions—an additional cost-saving feature. Recycling would also be significantly less problematic and lead to more sustainability.
Journal Reference:
Liu, Z., et al. (2024) An Ultralow-concentration and Moisture-resistant Electrolyte of Lithium Difluoro(oxalato)borate in Carbonate Solvents for Stable Cycling in Practical Lithium-ion Batteries. Angewandte Chemi. doi.org/10.1002/anie.202400110
Source: https://onlinelibrary.wiley.com/