Sponsored by HORIBADec 16 2013
Using fluorescent molecules known as molecular rotors is beneficial in determining the local nanoscale viscosity in microheterogeneous systems as it just requires the measurement of their fluorescence lifetime. This measurement can be performed in a simpler manner using microscope systems as corrections for photobleaching do not need to be made, as may be needed if a fluorescence anisotropy measurement was performed. Using molecular rotors is shown using the HORIBA DeltaMyc to monitor the gelation of a polysaccharide film.
Polysaccharide Films Made from Gellan Gum
Polysaccharides are biologically important polymeric molecules, in which repeat units of monomers are linked via glycosidic bonds. These carbohydrates play a key role in plant and animal nutrition and structure. Gellan gum comprises of a linear tetrasaccharide repeating unit and this versatile polysaccharide has found several applications, ranging from the food industry to drug delivery and tissue engineering applications. On heating, the helical polysaccharide backbone goes through a helix to coil transition accompanied by a significant change in viscosity.
A sol to gel transition is produced by subsequent cooling at 33 to 34°C, which is close that that of biological interest. There have been a number of reports studying its viscosity behavior over several concentrations and on addition of metal ions as cations help to crosslink gellan’s negatively charged polysaccharide helices, forming helix-helix aggregates. Normally studies on its viscosity determine the bulk property but as there is a growing interest in its biological activity, this has resulted in a need to examine changes on the microscope scale.
Molecular Rotors
A key feature of fluorescence is the sensitivity of a fluorophore to its microenvironment. Normally a fluorescence anisotropy measurement, where rotational molecular diffusion is monitored, maybe considered, but there are fluorophores that undergo a viscosity dependent intermolecular rearrangement that effects their fluorescent lifetime. This makes the decay acquisition faster by bringing down the number of measurements and in the case of microscopy, avoids problems that are associated with photo bleaching. An instance of such a molecule made use of this work.
Figure 1. The Stilbenoid Dye DASPMI
For using this phenomenon, a calibration is made by determining their fluorescence lifetimes in solutions of known viscosity (this should produce a linear calibration on a log-log plot of lifetime vs viscosity). An example of the fluorescence decays, measured on a HORIBA DeltaPro, and a plot for the average lifetime of DASPMI at different viscosities is shown in Figure 2.
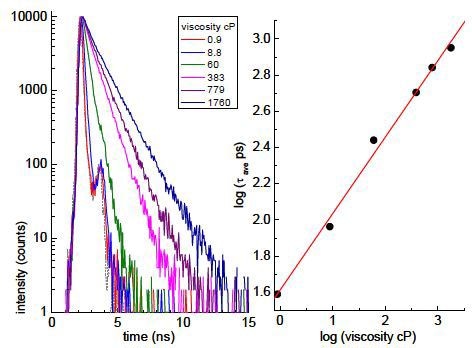
Figure 2. Plot for the average lifetime of DASPMI at different viscosities
Monitoring the Viscosity of Gellan Gum
The measurements were carried out using a DeltaMyc as shown in Figure 3 with FLIM (fluorescence lifetime imaging) performed making use of a 470nm emitting DeltaDiode laser running at 25MHz. A dye drop containing liquid gellan gum was arranged on a microscope slide and allowed to dry at ambient conditions. A drying sample is shown below in a recessed well on a slide.

Figure 3. DeltaMyc
An area across the drying front was selected for imaging and shown in Figure 4. The corresponding FLIM measurement is shown in Figure 5.
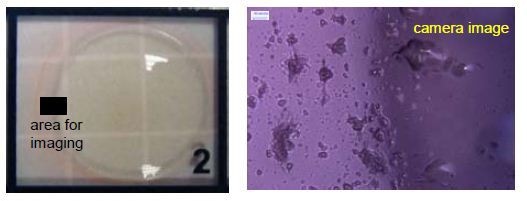
Figure 4. Area across the drying front
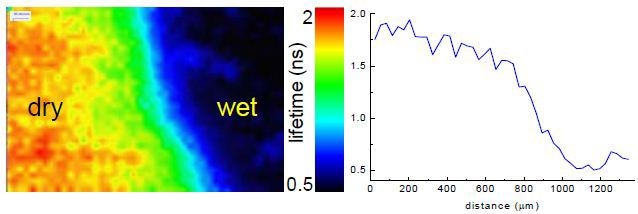
Figure 5. Corresponding FLIM measurement
This shows a decrease in the average lifetime, illustrated using a rainbow scale, on going from the drier region to the wetter one (left to right, with example cross-section). This corresponds to decreasing viscosity. Using the lifetime “calibration” enables the estimation that the viscosity increased ~600 times from the wet to dry region. It is also possible, using the microscope, to monitor the fluorescence lifetime of DASPMI at a single point, as the gellan gum film dries.
Examples of the fluorescence decays obtained are provided in Figure 6.
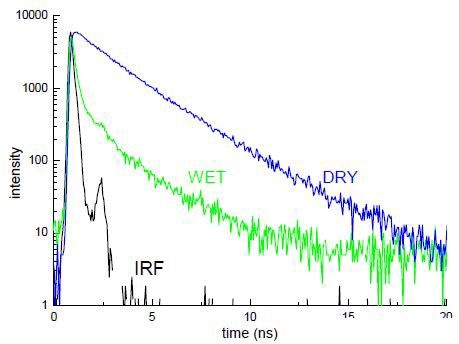
Figure 6. Example of fluorescence decays
This shows decays from which the average lifetime can be obtained using DAS6, after reconvolution with the instrumental response (IRF), which was obtained via scattering light from the microscope slide.
Conclusion
It can be observed from the time-resolved measurements made on the compact DeltaMyc that using fluorescence lifetime measurements it is possible to spatially monitor changes in viscosity associated with a gelation and drying process. The results shown in this note are based on the following the paper, G. Hungerford, M. Toury, D. McLoskey, N. Donaldson, A.S. Holmes-Smith, 2012. “In-situ formation of silver nanostructures within a polysaccharide film and its application as a potential biocompatible fluorescence sensing medium”, Soft Matter, 8, 653-659.

This information has been sourced, reviewed and adapted from materials provided by HORIBA.
For more information on this source, please visit HORIBA.