Free radicals are increasingly reactive chemical species that govern several fundamental chemical processes in nature, most notably combustion and oxidation. Till now direct measurement of the composition and concentration of free radicals has signified a challenge for chemists due to the complexity and expense of the necessary equipment. The Micro-ESR an innovation in sensor design determines free radicals with a low-cost, compact and ruggedized device.
New low-cost applications are enabled by the spectrometer such as online measurement of lubricant breakdown in engines and machinery, online airborne particulates monitoring in diesel engine exhaust and even spin immunoassay medical diagnostics.
Electron Spin Resonance
An electron spin resonance (ESR) spectrometer identifies the concentration and composition of free radicals present in a sample. Free radicals are molecular or atomic species with unpaired electrons which are usually highly reactive. The sample is loaded into a high-frequency resonant cavity in a slowly varying uniform magnetic field. Unpaired electrons irradiated with microwave radiation at a fixed frequency will undergo resonant transitions between the spin- up and spin-down state at a characteristic magnetic field governed by Equation 1, shown conceptually in Figure 2.

Figure 1. Micro-ESR Sensor is contained in a high-Q ceramic resonant
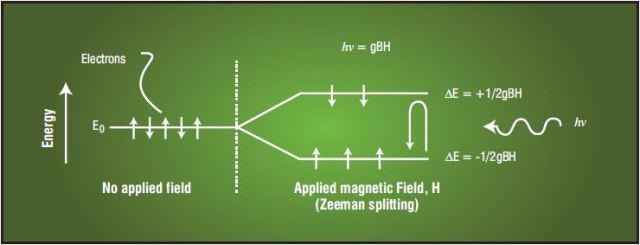
Figure 2. Electron Transitions Stimulated by Incident Microwave Energy
Here, h is Planck’s constant, β is the Bohr Magneton, ν is the resonant frequency, H is the applied magnetic field, and g is a characteristic of the radical (the g-factor, an empirically determined number, often close to 2.0000). The concentration of the radical in the sample determines the magnetic field at resonance is a function of the g-factor, and the height of the resonant peak.
The Micro-ESR features better sensitivity and cost reduction by a factor of 1,000 compared to conventional ESR spectrometers.
Table 1. Specifications
| Supply Voltage |
12-32 VDC |
| Sensor Output |
Options USB, Modbus®, RS-232, Ethernet, CAN. Additional communication standards are available according to customer specifications. |
| Dimensions: |
2.25-inch Φ by 1.5-inch tall cylindrical metal package with hydraulic and electrical connections. |
| Fittings |
7/16-inch JIC, NPT, others available according to customer specifications. |
| Quantities Measured |
Oxidation and soot (peroxy radical and carbon radical), water content, fuel dilution (marine applications only), RF dielectric permittivity. |
| Operating Temperature Range |
-30°C to +120°C |
| Max. Inlet Oil Temperature |
160°C+ |
Experimental Results
Initial experiments for verifying the spectrometer operation were conducted with DPPH, a dye containing a stable free radical, dissolved in toluene solvent. DPPH dissolved in toluene exhibits a characteristic set of resonant peaks that correspond to hyperfine splitting.
Engine oil from a Honda gasoline engine was studied. As the antioxidant package in the oil is depleted by oxidation, the intensity of the peroxy radical (RO2•) signal increases steadily from zero. This is the induction period. Also, the g-factor of the peroxy radical signal increases slightly as the hydrocarbon chains are broken down.
When the oil is approaching the end of its useful life, the intensity of the peroxy radical signal increases dramatically and failure is imminent, as shown in Figure 3.
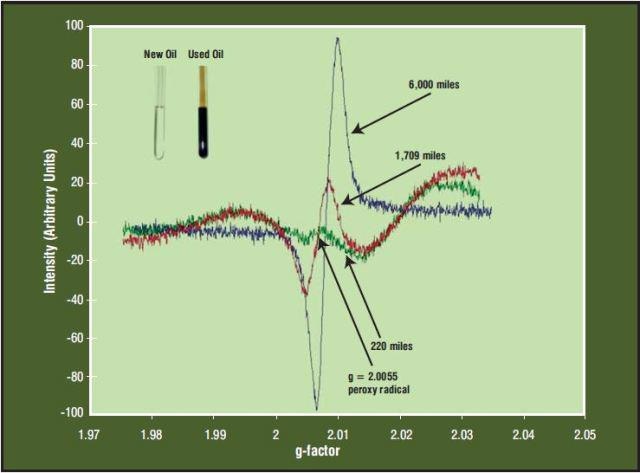
Figure 3. Peroxy Radical vs Mileage
The oxidation chain reaction can be examined in more detail to understand the importance of the peroxy radical in lubricant breakdown. First, free radicals are produced by exposing oil to high temperatures in the presence of oxygen:

The chain reaction then propagates as:
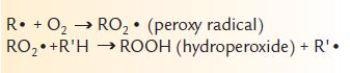
Normally, antioxidants are added to the base oil, which react with the peroxy radical and render it harmless. However, as the antioxidants in the oil are consumed, the concentration of peroxy radicals increases and breakdown accelerates.
In Figure 4, all of these compounds can be observed experimentally. Cylinder lube oil is used typically once and then burned in the engine. Lube oil feed rates must also be adjusted depending on the concentration of sulfur in the fuel oil, and measurement of the sulphur content of the fuel is of interest to ship operators who seek to minimize operating costs.
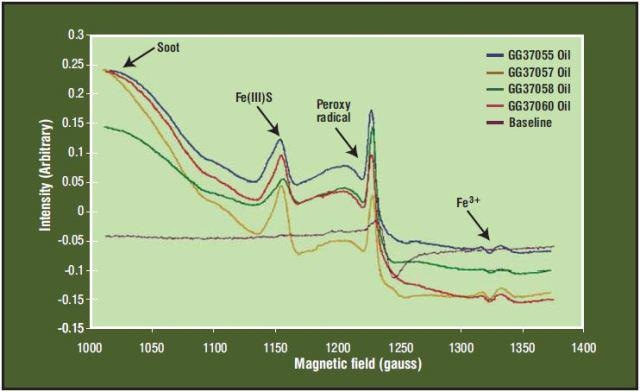
Figure 4. ESR Spectra of Marine Engine Cylinder Lube Oil Samples
Applications
The examples above clearly show the breadth of applications (100-hp gas motors to 75,000-hp marine diesels) where the sensor can be applied. As seen in Figure 4, carbon soot particulates can be readily detected by the spectrometer, both when dissolved in the oil and also from airborne soot. This enables its use to determine the composition and concentration of airborne soot particulates in vehicle or powerplant emissions. Just as an oxygen sensor in a gasoline engine is used to adjust the fuel-air mixture to prevent the engine from running too rich, an electro spin resonance airborne soot sensor can be used for adjusting the fuel-air mixture in a diesel engine to prevent excess particulate emissions. This is a very important application as new emissions standards are continually challenging vehicle manufacturers to reduce particulate emissions.
Competitive Advantages
This spectrometer determines intrinsic chemical properties of the oil (concentration of chemical constituents), while other approaches measure physical properties of the oil (such as dielectric constant, viscosity, electrical impedance) and then relate that data to underlying chemical changes in the oil.
Another benefit is that the spectrometer gives an absolute reading of the condition of the oil. New oil has a null spectrum i.e there are no free radicals, carbon or other contaminants present in the oil. The presence of any ESR spectrum clearly shows that contamination is present in the oil.
In addition, the g-factor as in Equation 1 of each free radical is only weakly dependent on temperature. because free radicals in oil can be uniquely identified by their g-factors, this enables the user to easily identify any ESR signals with absolute confidence at any operating temperature. The specificity of ESR implies that no compounds other than free radicals or transition metal ions will produce a signal. The technique, therefore, does not exhibit crossfactors commonly seen with other sensors.
This application of the spectrometer offers the end-user a new and cost-effective method to ensure compliance with environmental standards. It also enables the user to identify when an engine is running too rich, which severely reduces fuel efficiency.
Conclusion
Micro-ESR signifies a fundamental advance in the state-of-the-art of chemical sensor technology. Despite the enormous breadth of applications of electron spin resonance spectrometry, there have been no fundamental advances in the core design of the spectrometer until now.

This information has been sourced, reviewed and adapted from materials provided by Bruker BioSpin - NMR, EPR and Imaging.
For more information on this source, please visit Bruker BioSpin - NMR, EPR and Imaging.