A structure for “quality by design” deployment is provided by the FDA’s process analytical technology (PAT) initiative to gain insights into industrial pharmaceutical processes in real time and in situ using analyzer technologies. This knowledge is valuable in the development of rational specifications, critical quality attributes, and effective control limits.
Instrumentation
Raman spectroscopy is ideally suited for PAT applications, thanks to its ability to perform off-line laboratory analysis as well as in-line and on-line process analysis. Kaiser Optical Systems’ RamanRxn Systems line of Raman analyzers is ideal for monitoring each drug development process, staring from discovery to manufacture.
Sample preparation is not required for Raman spectroscopy, whereas other methods demand extraction of excipients and plasticizers. This experiment used Kaiser Optical Systems’ RamanRxn1™ analyzer coupled to a MR Probe Head and a 2.5-inch non-contact optic.
Experimental Procedure
Hot-melt extruded formulations are gaining traction for topical delivery of therapeutic compounds. This experiment analyzed an extruded polyethylene oxide (PEO) film consisting of ketoprofen in a lab environment as well as in a process line.
Off-Line Analysis
The MR Probe Head and the non- contact optic were focused on the extruded film to perform the off-line analysis using an exposure time of 30s for each spectrum. The ketoprofen samples’ unprocessed off-line Raman spectra are shown in Figure 1.
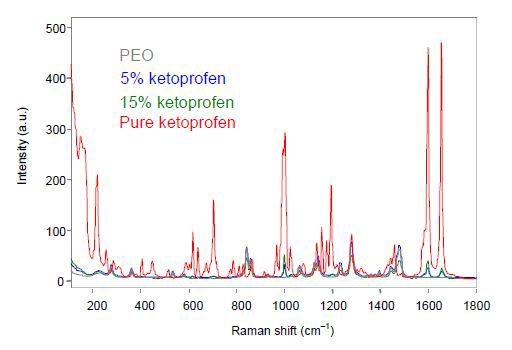
Figure 1. Off-line Raman spectra of ketoprofen films.
The baseline variation in the data was removed by applying a second-derivative treatment. As shown in Figure 2, the key area of variance was in the wavelength rage of 985-1030cm-1.
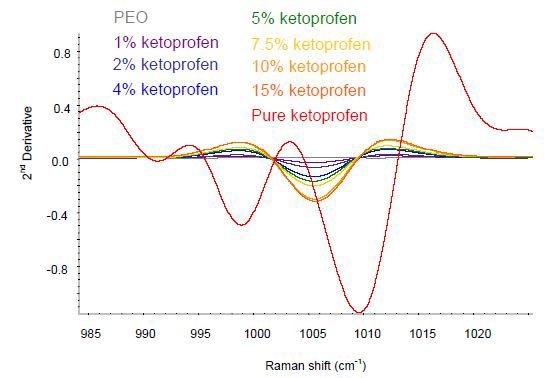
Figure 2. Second-derivative ketoprofen spectra in the spectral area of interest.
The extruded ketoprofen films show spectral changes corresponding to the crystalline pure sample and the spectral variations are in good agreement with the interpretation describing the transformation of the ketoprofen to amorphous when subjected to extrusion. Hence, Raman spectroscopy can provide quantitative information about the composition as well as qualitative information on the solid form of the sample.
Figure 3 presents the calibration results for off-line analysis of the ketoprofen films. The calibration coefficient for this dataset using a multiple linear regression (MLR) calibration with a primary wavelength shift of 998cm-1 and a secondary wavelength shift of 886cm-1 was calculated to be 0.9979. This means the Raman data are in good correlation with the known quantity of the ketoprofen content in the sample.
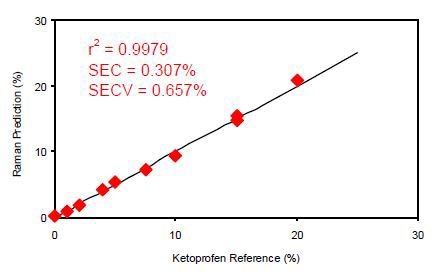
Figure 3. Calibration data for off-line ketoprofen measurements. SEC = Standard error of calibration; SECV = Standard error of cross validation.
On-Line Analysis
The same RamanRxn1 analyzer was used to perform the on-line analysis by collecting data during the extrusion of the film. The spectra were continuously collected by mounting the Raman probe over the moving film using an exposure time of 60s for each spectrum. Figure 4 shows the second-derivative spectra for the extruded ketoprofen film.
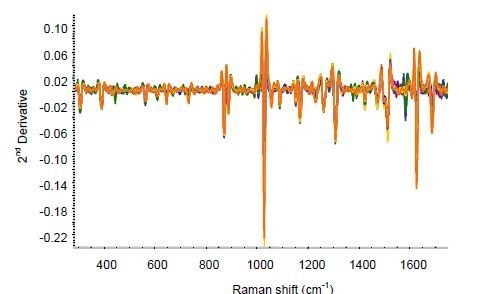
Figure 4. Second-derivative spectra for on-line ketoprofen measurements. Several spectra each are included for ketoprofen contents of 2.5%, 5%, 7.5%, 10%, 15%, and 20%.
In this case, partial least squares (PLS) models were applied to the Raman data due to the presence of more noise when compared to the offline measurements. Kaiser Optical Systems’ HoloPlex™ Advantage enabled simultaneous data acquisition across the whole spectral range devoid of scanning. This not only ensured accurate spectral collection from moving ketoprofen samples but also aided the application of the highly sensitive PLS models. Figure 5 presents the correlation curve.
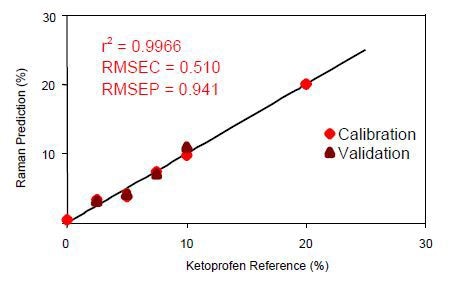
Figure 5. Calibration data for on-line ketoprofen measurements. RMSEC = Root mean squared error of calibration; RMSEP = Root mean squared error of prediction.
Using a three-factor PLS equation with a wavelength shift range of 506-1616cm-1, the correlation coefficient was calculated to be 0.9966. This value represents the excellent correlation of the Raman data to the known ketoprofen content in the sample.
Normalization of the data was then performed to the area of the peak between the range of 1409-1524cm-1. The PLS model results demonstrate the ability of the Raman system to perform accurate online measurements of ketoprofen concentrations during manufacturing.
Conclusion
The results demonstrate that Raman spectroscopy is ideal for this PAT application, enabling to gain knowledge about the process in real time. Both off-line and on-line Raman data are in good correlation with the known ketoprofen content in the sample, proving the suitability of the Raman system to analyze the samples off line in a laboratory environment as well as on-line process lines.
About Kaiser Optical Systems
Kaiser Optical Systems, Inc. is a world leader in spectrographic instrumentation and applied holographic technology. Principal products include Raman sensors and instrumentation, advanced holographic components for spectroscopy, and astronomy and ultra-fast sciences. Principal offices and the manufacturing facility are located in Ann Arbor, Michigan.
Their products and services are deployed throughout the world in such diverse applications as pharmaceutical and chemical manufacturing, nanotechnology, telecommunications, education, forensic science, deep-sea exploration, and astronomy. From particles smaller than a human hair to objects as large as planets, their products are providing our customers unique insights into both today’s as well as “age-old” questions.
Kaiser was founded in 1979 to meet the need for diffractive or holographic optics for the avionics market. Kaiser entered the spectroscopy market in 1990 with the introduction of the holographic notch filter. In 1993 Kaiser released their first Raman analyzer, the HoloProbe. In 2013, the company became part of the Endress+Hauser Group.
To better serve the European community, Kaiser opened a new subsidiary in Europe in 1998. Kaiser Optical Systems SARL is located in Lyon, France. Kaiser SARL supervises their distributor network within Europe.

This information has been sourced, reviewed and adapted from materials provided by Kaiser Optical Systems.
For more information on this source, please visit Kaiser Optical Systems.