Detergent packaging has undergone many changes during the past decade to address the constantly changing customer requirements and environmental concerns. Detergents in tablet form by directly compressing dry powders are one of the major advances in detergent packaging in the past 10 years. Detergent tablets are beneficial over dosage forms in many ways, including precise dosage, compactness, physical and chemical stability.
Surfactants in detergent formulations are responsible for the removal of water-soluble as well as non-water-soluble soils. The detergent industry is showing interest to incorporate surfactants in tablets without sacrificing end-use properties, including dissolution, physical and mechanical properties, during the storage period. This article presents the application of Raman spectroscopy to analyze the stability of detergent tablets under controlled storage conditions.
Experimental Procedure
Kaiser Optical Systems’ RamanRxn1™-based PhAT System™ equipped with a diode laser of 785-nm wavelength and an optimum power of 200mW was used to conduct the spectral analysis of powders. The PhAT System allows for non-destructive data acquisition using a non-contact probe. It is capable of collecting data from a spot having a diameter of roughly 3-6mm compared to approximately 60µm for an immersion probe, thereby alleviating the sub-sampling problem. Each molecule is determined by the characteristic vibrational frequencies of the molecule through spectral collection over the range of 150–1825cm–1.
This experiment used five components, including a chlorine provider (dihydrated sodium dichloroisocyanurate: DCCNa), a carbonate (sodium bicarbonate) coupled to an organic acid (adipic acid—dry recrystallized) for effervescence, a surfactant (sodium dodecyl sulfate: SDS), and a disintegrant (sodium croscarmellose) to manufacture detergent compacts at a controlled compaction ratio of roughly 90%.
Experimental Results
Raman spectra for tablets consisting of the surfactant SDS as well as for pure SDS when stored at different relative humidities are depicted in Figure 1. The characteristic surfactant peaks can be observed even for the low surfactant content (2%) of the tablets, corroborating the sensitivity and the applicability of the Raman analyzer. Moreover, the characteristic surfactant peaks remained constant at all humidities, indicating that the RH change had no effect on the surfactant.
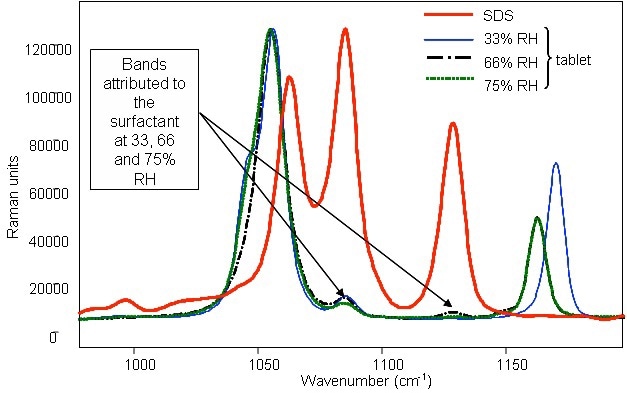
Figure 1. Raman spectrum of powder SDS and identification of the compound in detergent tablets with surfactant after storage in various relative humidities.
Figure 2 shows the characteristic peaks of sodium bicarbonate at 1044 and 1265cm–1, which were appeared at 33% RH. The 1044cm–1 band disappeared at75% RH and the position of the 1265cm–1 band shifted, revealing the change in the structure of the sodium bicarbonate with humidity irrespective of the presence or absence of the surfactant.
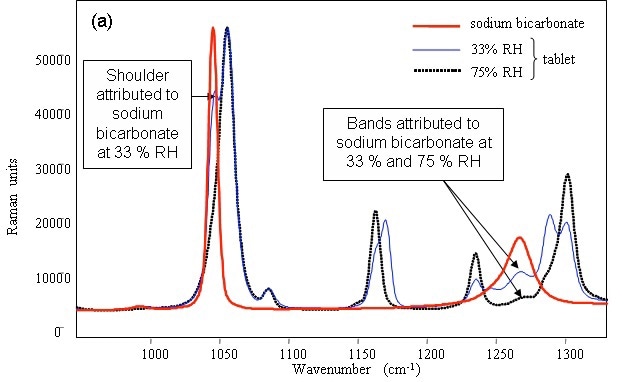
Figure 2. Raman spectrum of pure powdered sodium bicarbonate and identification of the compound in detergent tablets after storage in various relative humidities.
The characteristic band of the adipic acid sample remained constant at 915cm–1 when stored at 33% RH (Figure 3). However, the 915cm–1 band shifted to 930cm–1 when exposed to 75% RH, revealing the structural change in the compound. The band shift for both sodium bicarbone and adipic acid at 75% RH may be in agreement with a hypothesis describing the onset of effervescence during the exposure of tablets to high humidities above 75% RH.
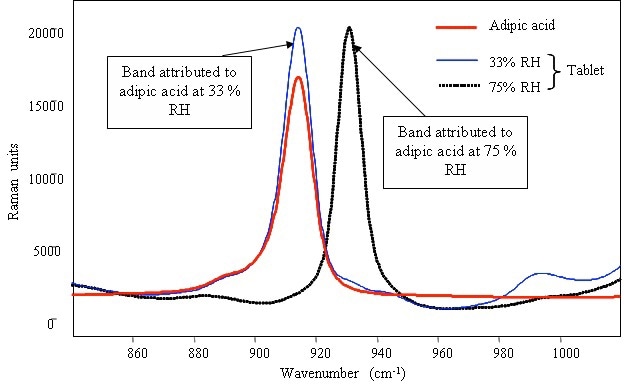
Figure 3. Raman spectrum of pure powdered adipic acid and identification of the compound in detergent tablets after storage in various relative humidities.
Figure 4 presents the characteristic peaks of DCCNa at 1155 and 1300 cm–1, showing corresponding shoulders at 1165 and 1280cm–1. The comparison of the corresponding intensity and position of the main peaks and their shoulders for the detergent tablets was performed at 33% and 75% RH. The tablets devoid of the surfactant showed different features at 33% and 75% RH. Although the positions of these peaks remained constant at 33% RH, there was a significant difference in their relative intensities when compared to pure DCCNa. Conversely, the position and relative intensity of the bands were in line with those of pure DCCNa.
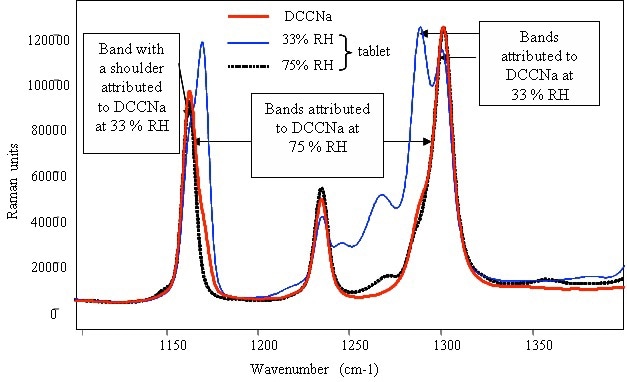
Figure 4. Raman spectrum of pure powdered DCCNa and identification of the compound in detergent tablets after storage in various relative humidities.
Conclusion
The results reveal that there was no transformation of the surfactant due to moisture, but effervescence was induced as indicated by the modifications of adipic acid and sodium bicarbonate at 75% RH. There was a band shift for the DCCNa in the tablets at 33% RH in the absence of the surfactant as well in the presence of surfactant at higher humidity, corroborating that DCCNa modifications took place later in the presence of surfactant. This observation is in good agreement with the conclusions arrived with the evaluation of finished tablets in the course of storage.
About Kaiser Optical Systems
Kaiser Optical Systems, Inc. is a world leader in spectrographic instrumentation and applied holographic technology. Principal products include Raman sensors and instrumentation, advanced holographic components for spectroscopy, and astronomy and ultra-fast sciences. Principal offices and the manufacturing facility are located in Ann Arbor, Michigan.
Their products and services are deployed throughout the world in such diverse applications as pharmaceutical and chemical manufacturing, nanotechnology, telecommunications, education, forensic science, deep-sea exploration, and astronomy. From particles smaller than a human hair to objects as large as planets, their products are providing their customers unique insights into both today’s as well as “age-old” questions.
Kaiser was founded in 1979 to meet the need for diffractive or holographic optics for the avionics market. Kaiser entered the spectroscopy market in 1990 with the introduction of the holographic notch filter. In 1993 Kaiser released their first Raman analyzer, the HoloProbe. In 2013, the company became part of the Endress+Hauser Group.
To better serve the European community, Kaiser opened a new subsidiary in Europe in 1998. Kaiser Optical Systems SARL is located in Lyon, France. Kaiser SARL supervises their distributor network within Europe.

This information has been sourced, reviewed and adapted from materials provided by Kaiser Optical Systems.
For more information on this source, please visit Kaiser Optical Systems.