The accessibility of at-line techniques, such as thin layer chromatography (TLC), gas chromatography- mass spectrometry (GC-MS), have left them routine practices in reaction monitoring. Despite the competitive advantages of NMR – including inherently quantitative data and relatively easy to solve spectra – traditional NMR instrumentation are not optimized for reaction monitoring. Super-conducting spectrometers are typically located off-line in centralized facilities that cannot necessary be accessed precisely when required. The Nanalysis 60 spectrometers, on the other hand, easily address these concerns. The affordable and compact footprint allows chemists to site the instrument directly at the point of need – on the bench, in the fumehood, or even in a glovebox and measure a variety of nuclei including 1H, 19F and 31P.
Additionally, the stable permanent magnetic field affords reproducible and reliable results that can be acquired over long intervals without requiring touch-up shims. Although there are many potential reactions that are suitable to low-field NMR spectroscopy, we have chosen to highlight a transesterification reaction.
Transesterification: Synthesis of Biodiesel
Sustainability is increasingly becoming a focus of innovation – with a rise in focus on green chemistry and renewable energy. Biodiesel is synthesized through the transesterification of oils, fats and greases with short chain alcohols like methanol (MeOH) or ethanol (EtOH). This improves the process-ability and usability characteristics of the organics (e.g., reduces the viscosity).
Oils are triglycerides of fatty acids. This means that each triglyceride molecule has three fatty acid chains that are composed by a combination of saturated (e.g., palmitic (C16:0), stearic (C18:0)), monounsaturated (e.g., oleic (C18:1)) and polyunsaturated (e.g., linoleic (C18:2), alpha-linolenic (C18:3)) fatty acids. Although not fully resolved at 60 MHz, there are distinctive chemical shift regions that can be used to differentiate the types of fatty acids. These are labeled on the spectra below: carboxylic acid (COOH) (δ 10.50 – 12.00), olefin (CH=CH) δ 4.92 – 5.67, bis-allylic (=CH-CH2-CH=) δ 2.50 – 3.05, allylic/alpha-C (=CH-CH2-alkyl or O=C-CH2) δ1.75 – 2.50, and alkyl (-CH2/-CH3)’ δ 0.46 – 1.75 ppm.
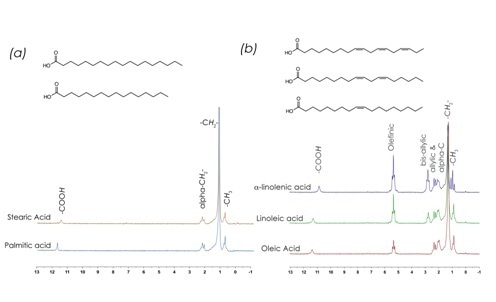
Oils, fats and greases will have similar 1H NMR spectra to the free fatty acids, but instead of the carboxylic acid resonance, there will be a glyceride resonance that is indicative of the triglyceride linker (– CH2- δ 3.82 – 4.5 ppm) and the resonance integrals will be dependent on the composition of each oil. Depending on the origin of feedstock used for biodiesel synthesis each batch will have a different composition of fatty acids, and these can be differentiated by integrated the relative regions.
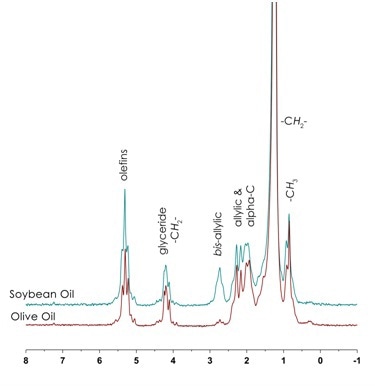
Typical reactions react the triglyceride oil with a base in the presence of the alcohol and mild heat. This forms glycerol out of the triglyceride linkage and the resultant ester, in the case study shown here we are using methanol to synthesize fatty acid methyl esters (FAMEs). In this case we expect to see the glyceride and methanol resonances to decrease in intensity as they are consumed and the methyl ester resonance to increase as we form biodiesel.
Results in Proteo- vs. Deuterated Solvents
To monitor this, a 100 µL aliquot was taken and dissolved in 400 µL of solvent (CDCl3) and a 1H NMR was measured until the reaction was assessed to have reached completion.
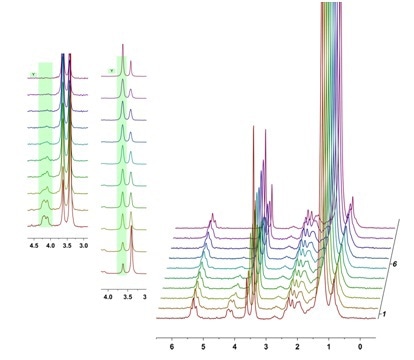
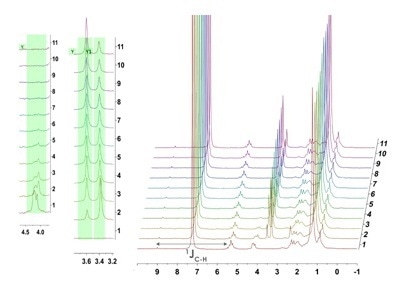
Alternatively, however, the 1H NMR could have been collected in CHCl3, a more affordable and industrial relevant solvent. The data collected is still within dynamic range, the 13C satellites of the strong solvent peak overlap slightly with the olefin peak but do not mask the transesterification reaction. This data can easily be used to generate speciation plots and determine rates of reaction.
Conclusions
The Nanalysis 60 is a powerful detector that can easily be used at-line for reaction monitoring of typical organic synthesis reactions in ways that traditional NMR instrumentation simply cannot.

This information has been sourced, reviewed and adapted from materials provided by Nanalysis Corp.
For more information on this source, please visit Nanalysis Corp.