Nuclear Magnetic Resonance (NMR) spectroscopy is a useful tool for exploring various domains across the natural sciences. NMR is well-suited to the analysis of molecular properties, such as the amide bond, which has a limited rotation around the C–N bond. In biochemistry, the amide bond is commonly known as the peptide bond. This bond is brought about by the union of a carboxyl group of one amino acid with the amino group of another amino acid. Simply put, a dehydration reaction allows the two amino acids to form a peptide bond (as shown in Scheme 1).
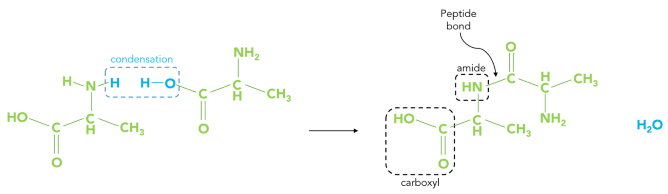
Scheme 1. Dehydration reaction between two residues to form a longer amino chain and water as a by-product. Image Credit: Nanalysis Corp.
In the amide, N,N–dimethylacetamide (DMA), two different signals are observed on the 1H NMR spectrum as two methyl groups bond to the nitrogen (Figure 1). Resonance stabilization should be evaluated to better understand this.
Resonance stabilization refers to the process where electrons are delocalized when surrounded by a number of atoms (Scheme 2). In the case of amides, this produces a hybrid structure that leads to the C–N bond having some double-bond character; hence, rotation is limited. At room temperature, a difference in the chemical composition results in the methyl groups bonded to the nitrogen in DMA due to the contribution of the double character.
At increased temperatures, the energy barrier to rotation is circumvented, and a rapid rotation of the C–N bond is observed. This fosters an environment suitable for both N–methyl groups.
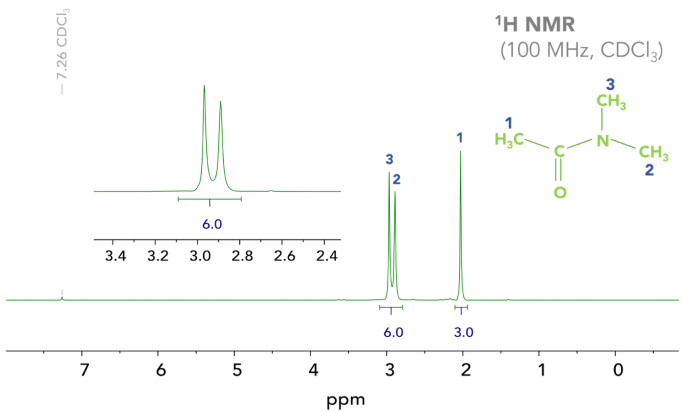
Figure 1. 1H NMR Spectrum of DMA, demonstrating that the two N–methyl groups have different chemical shifts. Image Credit: Nanalysis Corp.
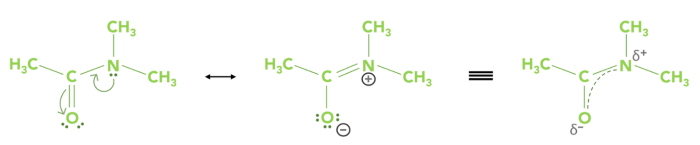
Scheme 2. Resonance structures of DMA, the hybridization results in the delocalization of electrons (indicated by the dotted line) between the O–C–N bonds. Image Credit: Nanalysis Corp.
To summarize, NMR is useful for the study of the molecular properties of compounds. This can support various interpretations of the compounds of interest and helps generate a deeper understanding of advanced chemical concepts. For instance, dynamic processes that include rotational barriers and any conformational shifts of cis-trans isomers.1
References and Further Reading
- Huggins, M.T.; Kesharwani, T.; Buttrick, J.; Nicholson C. J. Chem. Educ. 2020, 97, 1425 – 1429
- https://researchguides.library.vanderbilt.edu/c.php?g=69346&p=449918 (accessed February 2023) - Vanderbilt University

This information has been sourced, reviewed and adapted from materials provided by Nanalysis Corp.
For more information on this source, please visit Nanalysis Corp.