Most students know about aspirin, a pain-relieving compound, and they gain knowledge about the role of chemistry in real-life applications from its synthesis. It is possible to perform aspirin synthesis in a single step, such as O-acetylation of salicylic acid (Figure 1), which is part of many undergraduate synthetic chemistry laboratory courses.
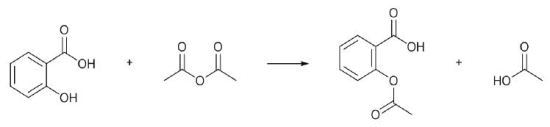
Figure 1. O-Acetylation of salicylic acid to give acetylsalicylate (aspirin)
Aspirin synthesis may also include an additional step, in which wintergreen oil (methyl salicylate) is converted into salicylic acid (Figure 2). This helps students to gain an understanding about multi-step synthesis and the idea of transforming a naturally occurring substance into a therapeutically valued product. As a result, students also have an additional compound for isolation, purification, determination of yield, and characterization by 1H-NMR spectroscopy.
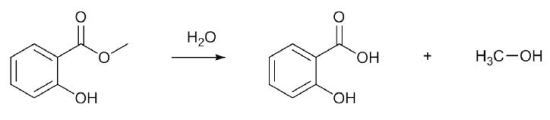
Figure 2. Hydrolysis of methyl salicylate (wintergreen oil) to give salicylic acid
Significance of Determining Aspirin Purity
The purity of the product is essential for its use as a drug, thus necessitating students to measure the purity of their products. Thin layer chromatography (TLC) is normally used for this purpose. This article discusses the use of 1H-NMR spectroscopy in a conventional undergraduate organic chemistry experiment so that students will get the exposure to report the chemical shifts of a synthetic product in addition to measuring the purity of their sample.
In this laboratory, students can learn about the facets of practical organic chemistry, including synthesis, stoichiometry, crystallization, and percent yield. For this purpose, 1H-NMR spectra have to be obtained for their starting materials, crude and purified products.
Synthesis of Salicylic Acid from Wintergreen Oil
Hydrolysis of wintergreen oil is shown in Figure 3 and summarized in Table 1.

Figure 3. Hydrolysis of wintergreen oil
Table 1. Synthesis of salicylic acid from oil of wintergreen
| |
Methyl Salicylate |
Salicylic Acid |
| Molecular Mass |
152.15 g mol-1 |
138.12 g mol-1 |
| Stoichiometry |
1 |
1 |
| Density |
1.17 g mL-1 |
Solid |
| Moles |
0.03 mol |
Theoretical: 0.03 mol |
| Mass |
4.68 g (4 mL) |
Theoretical: 4.14 g |
Procedure
Around 4 mL of methyl salicylate and 40 mL of 6 M sodium hydroxide are added into a beaker, and then stirred. The mixture is gently boiled by heating with occasional stirring for 15 min. Solids from the beaker sides are washed away using a small amount of distilled water during heating. The reaction mixture is subsequently cooled down in an ice bath to reach a temperature that is warm to touch.
Keeping the beaker in the ice bath, around 50mL of 8 M sulfuric acid is added to the reaction mixture with simultaneous stirring. The mixture in the ice bath is allowed to chill to form crystals. Buchner filtration is used to separate the precipitate and the resulting solids are then rinsed with a small amount of cold distilled water. Now, a yield and 1H-NMR spectrum (in chloroform-d) of the crude product are obtained (Figures 4 and 5).
The crude product is recrystallized from the distilled water and the resulting crystals are then dried using a desiccators (Figure 4). Now, a yield and 1H-NMR spectrum (in chloroform-d) of the purified product are obtained. The 1H-NMR spectrum (in chloroform-d) of methyl salicylate, the starting material, is also recorded (Figure 5).

Figure 4. Crude and recrystallized salicylic acid
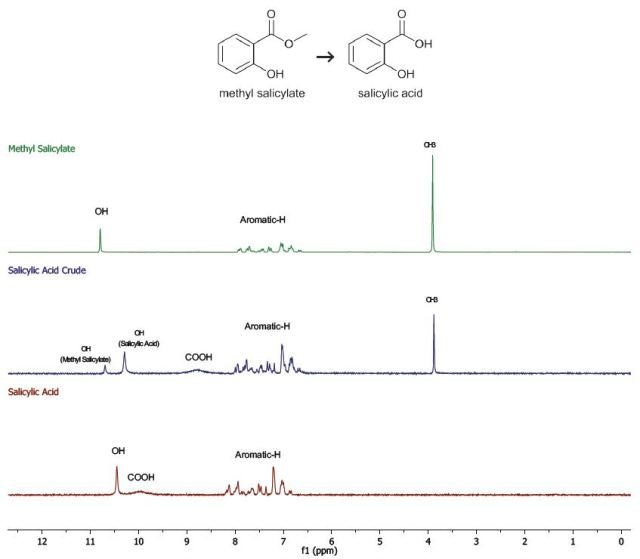
Figure 5. The 1H-NMR spectra of the products
Risk Assessment
Besides being combustible, methyl salicylate and salicylic acid are harmful when swallowed. Sodium hydroxide causes severe burns as it is corrosive and alkaline. The 6 M solution needs to be prepared carefully because of generation of heat due to exothermic dissolution of sodium hydroxide pellets. Sulfuric acid is highly corrosive, causing severe burns. Therefore, the 8 M solution needs to be carefully prepared by adding the acid to the water, and not the other way around.
Synthesis of Aspirin from Salicylic Acid
Figure 6. and Table 2 show aspirin synthesis from salicylic acid.

Figure 6. Acetylation of salicylic acid
Table 2. Synthesis of aspirin from salicylic acid
| |
Salicylic Acid |
Acetic Anhydride |
Sodium Acetate |
Acetylsalicylic Acid |
Acetic Acid |
| Molecular Mass |
138.12 g mol-1 |
102.09 g mol-1 |
82.03 g mol-1 |
180.16 g mol-1 |
60.05 g mol-1 |
| Stoichiometry |
1 |
1 (3, excess) |
(Catalyst) |
1 |
1 |
| Density |
Solid |
1.08 g mL-1 |
Solid |
Solid |
1.05 g mL-1 |
| Moles |
0.014 mol |
0.042 mol |
0.0047 mol |
Theoretical: 0.014 mol |
Theoretical: 0.014 mol |
| Mass |
2 g |
4 g (4.4 mL) |
0.4 g |
Theoretical: 2.52 g |
Theoretical: 0.84 g (0.8 mL) |
Procedure
Around 0.4 g of anhydrous sodium acetate is added to a suspension of 2 g of salicylic acid in 4.5 mL acetic anhydride in a conical flask with simultaneous stirring. The reaction mixture is heated for roughly 15 min. After the dissolution of the solid, the heat is removed and 20 mL of distilled water is added. The flask is now placed in an ice bath to cool down the mixture until the formation of crystals.
Buchner filtration is used to collect the precipitate and the resulting solids are rinsed with cold distilled water. Now, a yield and 1H-NMR spectrum (in chloroform-d) of the crude product are obtained. The crude product is recrystallized from distilled water and the resulting crystals are then dried in a desiccator. Now, a yield and 1H-NMR spectrum (in chloroform-d) of the purified product are obtained. The 1H-NMR spectrum (in chloroform-d) of the starting materials acetic anhydride and salicylic acid (when the first step of synthesis is not performed) is also recorded (Figures 7 and 8).

Figure 7. Crude and recrystallized aspirin.
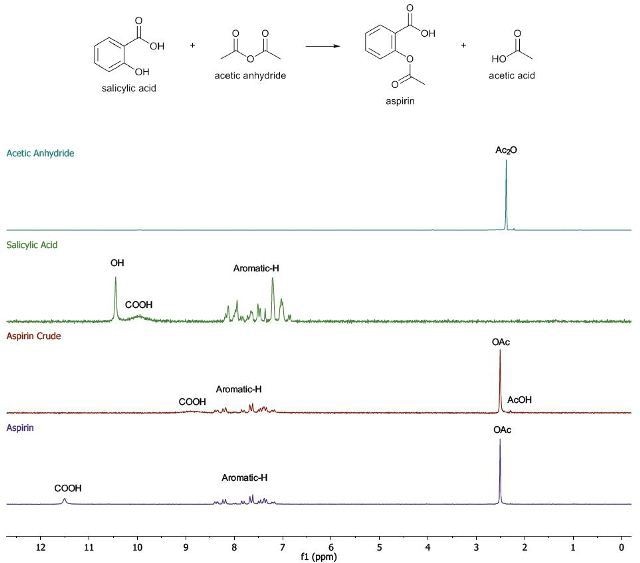
Figure 8. The 1H-NMR spectra of the products for aspirin synthesis from salicylic acid
Risk Assessment
Besides being combustible, methyl salicylate and salicylic acid are harmful when swallowed. Ethanol is a highly flammable liquid, and acetic anhydride is highly flammable and corrosive, thus causing severe burns.
This information has been sourced, reviewed and adapted from materials provided by Magritek.
For more information on this source, please visit Magritek.