NMR spectroscopy is very useful for identifying the chemical structure of compounds. It is not just capable of offering data with regards to the functional groups present but also offers data about where the atoms are located in the molecule. This article explains how NMR is used for differentiating between structural isomers with the chemical formula, C4H8O2.
Student Instructions
The three isomers discussed have unique carbon skeletons and functional groups. All the three isomers are in liquid state at room temperature. Firstly, the 1D proton spectra of neat samples are determined on the Spinsolve® Proton NMR spectrometer. Sample preparation is performed by pipetting around 700µL of liquid into a 5mm NMR tube. The 1D proton spectrum of each compound is detected and the resonances in the spectrum are assigned to the structure.
Firstly, it is beneficial to understand the structure of the three isomers and ponder about the manner in which the 1D proton NMR spectrum will appear. Multiplicity, chemical shift, integration and the number of environments are the factors that need to be considered. Once the number of proton environments anticipated is ascertained, a table is developed with integration, splitting and chemical shift for understanding what the spectrum will appear like. Then using these forecasts, it can be recognized as to which spectrum belongs to each isomer.

Figure 1. Chemical structures of Isobutyric Acid, Ethyl Acetate and Butyric Acid
Example Discussion
Ethyl acetate

The number of environments observed in spectrum 3 (1, 2, 2')
Table 1. Chemical shift table for ethyl acetate
| Position |
Integration |
Splitting |
Chemical shift |
| 1 |
2 |
Three nearest neighbours that are equivalent – quartet |
Single bond to oxygen - deshielded |
| 2 |
3 |
Two nearest neighbours that are equivalent – triplet |
Saturated alkyl group far from ester group – shieldedt |
| 2' |
3 |
No nearest neighbours - singlet |
Adjacent to the carbon of a carbonyl -slightly deshielded |
Butyric acid
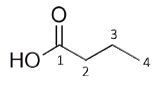
The number of environments observed in spectrum: 3 or 4 (2, 3, 4, possibly OH)
Exchangeable protons like the OH of a carboxylic acid are at certain times not present in the proton NMR spectrum since they exchange on the NMR experiment’s timescale.
Table 2. Chemical shift table for butyric acid
| Position |
Integration |
Splitting |
Chemical shift |
| 2 |
2 |
Two nearest neighbours that are equivalent –triplet |
Adjacent to the carbon of a carbonyl -slightly deshielded |
| 3 |
2 |
Five nearest neighbours in two different environments -quartet of triplets or triplet of quartets (will be observed as a multiplet) |
Saturated alkyl group far from carboxylic acid group - shielded |
| 4 |
3 |
Two nearest neighbours -triplet |
Saturated alkyl group far from carboxylic acid group - shielded |
| OH |
1 |
Exchangeable proton - singlet |
Proton directly bound to an oxygen -highly deshielded |
Isobutyric Acid

Number of environments observed in spectrum: 2 or 3 (2,3, possibly OH)
Table 3. Chemical shift table for isobutyric acid
| Position |
Integration |
Splitting |
Chemical shift |
| 2 |
1 |
Six nearest neighbours that are equivalent - septet |
Adjacent to the carbon of a carbonyl -slightly deshielded |
| 3 |
6 - equivalent methyl groups |
One nearest neighbour -doublet |
Saturated alkyl group far from carboxylic acid group - shielded |
| OH |
1 |
Exchangeable proton - singlet |
Proton directly bound to an oxygen -highly deshielded |
Spectrum 1, 2 and 3
There is no OH peak in spectrum 1 shown in Figure 2 though that does not mean that there isn’t an OH. However, since the integration values of the observed protons total to 8, it can be inferred there is no OH in the molecule. There is one small downfield resonance that looks like a quartet and there are two methyl groups that seem like a singlet and a triplet. These observations are in agreement with assigning the molecule as ethyl acetate.

Figure 2. Spectrum 1
Both spectra 2 and 3 (Figures 3 and 4) include a carboxylic acid’s OH peak. There are three chemical environments in spectrum 2, a septet, a doublet and the OH. This is in agreement with isobutyric acid predictions.

Figure 3. Spectrum 2

Figure 4. Spectrum 3
There is a carboxylic acid OH peak and several peak clusters between 0 and 2 ppm that characterize an alkyl chain in spectrum 3. Even though it seems complicated, three environments with very small differences can be observed. The upfield chemical shift of these three protons is in agreement with butyric acid predictions.
Conclusions
Taking into account the predictions made for the multiplicity of these protons, it can be suggested that the cluster of peaks are a multiplet and two triplets.
Ethyl acetate: Spectrum 1

1H NMR (42.5 MHz, neat) δ 3.45 (q, J = 7.1, 2 H, H-1), 1.36 (s, 3 H, H-2’), 0.60 (t, J = 7.1, 3 H, H-2).
Isobutyric acid: Spectrum 2

1H NMR (42.5 MHz, neat) δ 11.90 (s, 1 H, OH), 2.13 (sept, J = 6.8, 1 H, H-2), 0.73 (d, J = 6.7, 6 H, H-3).
Butyric acid: Spectrum 3

1H NMR (42.5 MHz, neat) δ 10.02 (s, 1 H, OH), 2.1-1.6 (m, 2 H, H-2), 1.6-0.8 (m, 2 H, H-3), 0.8-0.2 (m, 3 H, H-4).
This information has been sourced, reviewed and adapted from materials provided by Magritek.
For more information on this source, please visit Magritek.