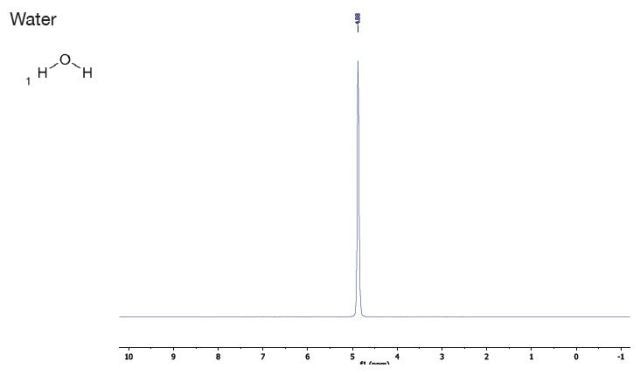
Final-year high school chemistry students undertaking an analytical instrumentation laboratory course at RMIT in Melbourne, Australia performed this experiment using the Spinsolve. This laboratory experiment has been adapted from material provided kindly by Dr Jeff Hughes to help introduce undergraduates to NMR spectroscopy.
Objectives
As a part of this experiment, students used 1H-NMR spectroscopy for identifying the key organic compounds present in typical household products, such as nail polish remover, vinegar and spirits. The spectra of common laboratory solvents and the spectra of household products are compared in order to determine the compounds.
Experiment
The students were assigned one reference solvent and one household product from the below list. Add around 0.5 mL of each liquid to a 5mm NMR tube. The 1H-NMR spectrum is determined for each of the samples. The spectra are phased and the peaks are integrated. The data obtained from the NMR spectrum of the reference solvent are recorded in a table.
In six groups, one for each household product and reference solvent, the organic compounds that are seen in each household product are identified. Finally, the volume percentage composition of every component is determined.
| Household product: |
Reference solvent: |
| Nail polish remover (pink) |
Acetone |
| Nail polish remover (blue) |
Ethyl Acetate |
| Rubbing alcohol |
Ethanol |
| Vinegar |
Acetic Acid |
| Vodka |
Water |
| Methylated Spirits |
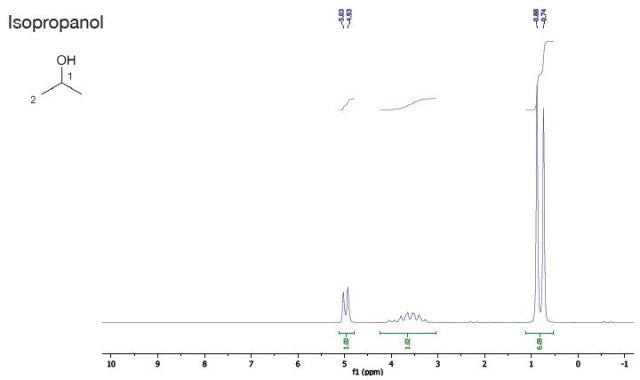
Isopropanol |
Safety
All the liquids feature a very high flammability and proximity to open flames and heat. Acetic acid causes corrosion, and may allow burns to skin and probably even eye damage.
Questions
1. What are the compounds that can be detected by the comparison of the household product spectra with the reference spectra?
2. Is the determined composition the same as that on the bottle label? In products that have one compound, is this key element mentioned on the bottle?
3. What compound properties that you detected rendered them useful in the product in which they have been included?
Example Spectra
Reference solvents
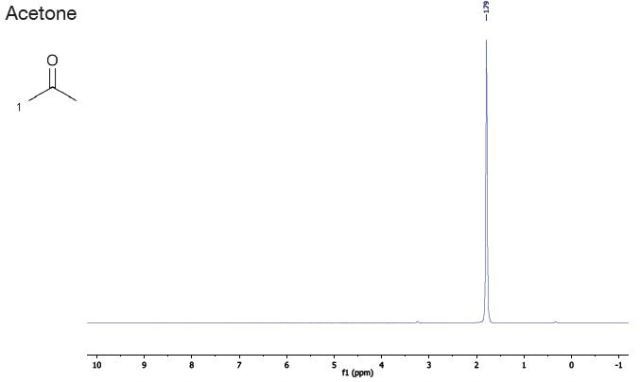
| Chemical Shift (ppm) |
Integration |
Multiplicity |
Assignment |
| 1.8 |
- |
singlet |
1 |
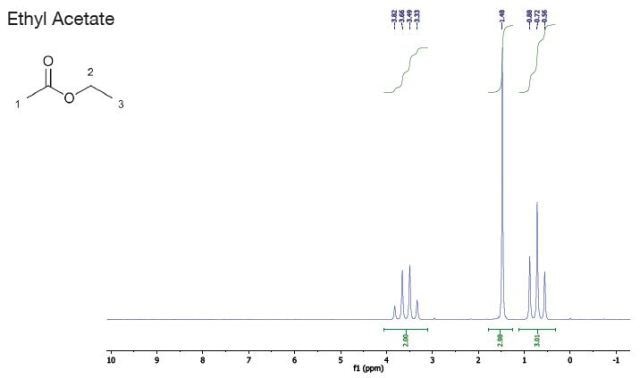
| Chemical Shift (ppm) |
Integration |
Multiplicity |
Assignment |
| 3.6 |
2 |
quartet |
2 |
| 1.5 |
3 |
singlet |
1 |
| 0.7 |
3 |
triplet |
3 |
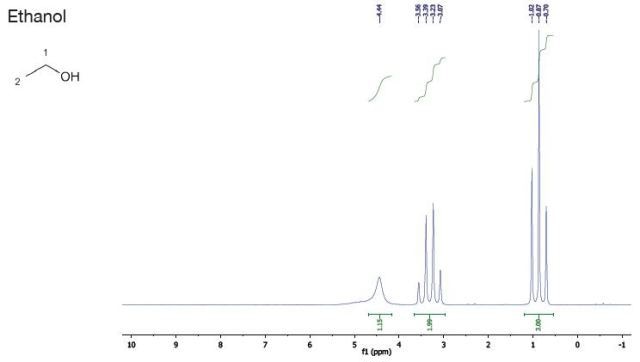
| Chemical Shift (ppm) |
Integration |
Multiplicity |
Assignment |
| 4.5 |
1 |
singlet |
OH |
| 3.3 |
2 |
quartet |
1 |
| 0.9 |
3 |
triplet |
2 |
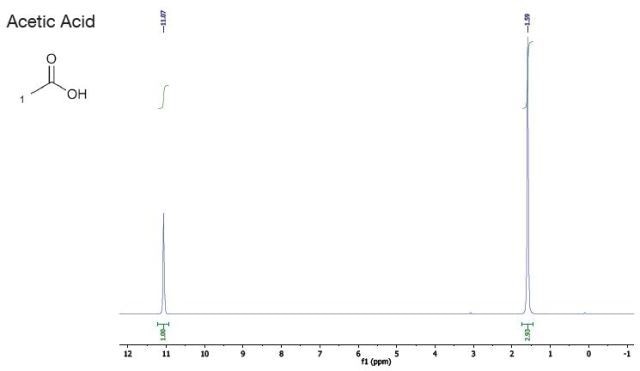
| Chemical Shift (ppm) |
Integration |
Multiplicity |
Assignment |
| 11.1 |
1 |
singlet |
OH |
| 1.6 |
3 |
singlet |
1 |
| Chemical Shift (ppm) |
Integration |
Multiplicity |
Assignment |
| 4.9 |
- |
singlet |
1 |
| Chemical Shift (ppm) |
Integration |
Multiplicity |
Assignment |
| 5.0 |
1 |
doublet |
OH |
| 3.6 |
1 |
multiplet |
1 |
| 0.8 |
6 |
doublet |
2 |
Household Products
Pink Nailpolish Remover
The integrals observed in the above spectrum make it obvious that the pink nail polish remover sample has more than one compound. It is observed from the spectrum that the peaks are in regions almost the same as ethyl acetate with the peak integral at 1.6 ppm, seemingly a single compound was fixed to 3 (ethyl acetate methyl).
If one compound is ethyl acetate, the other compounds’ chemical shifts are almost the same as the ethyl chain of ethyl acetate. This, along with an exchangeable proton at 4ppm, indicates that the other compound is most probably ethanol.
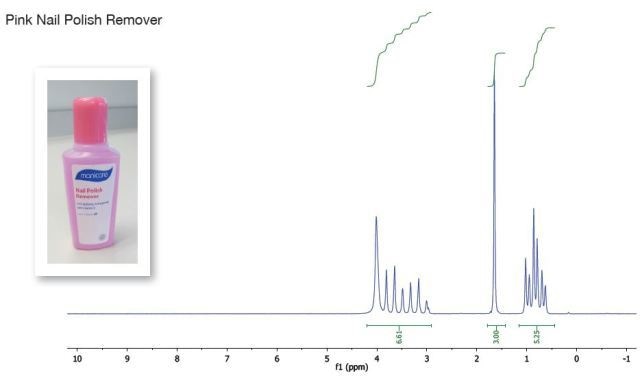
Figure 1. Pink nailpolish remover
For validating the assignments, the superposition of the spectra was performed in Mnova below. This exercise clearly elucidates the splitting of two methylene quartets and methyl triplets of the ethyl chain of ethanol and ethyl acetate.
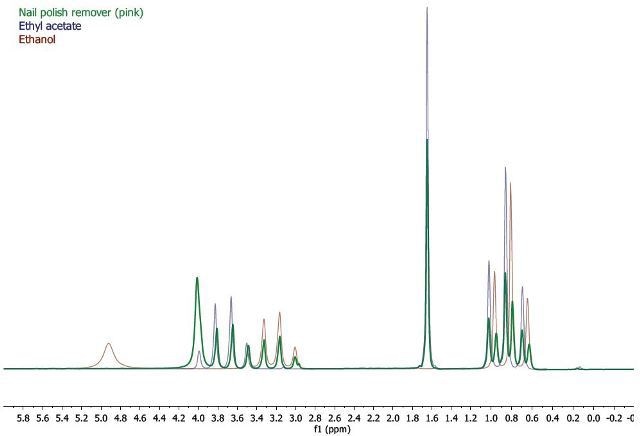
Figure 2. Splitting of two methylene quartets and methyl triplets
Percentage Composition
The singlet at 1.7 ppm characterizes the methyl close to the carbonyl of ethyl acetate, therefore accounting for three protons. Normalization of the rest of the integrals was performed based on this fact. Ethyl acetate’s other methyl is more up-field, and is in line with the methyl group of ethanol.
Three of the 5.25 protons correlate with the methyl of ethyl acetate since the normalization of the integrals was performed to the 1.7 ppm singlet. Hence, the rest of the 2.25 protons are the methyl of ethanol. The exchangeable protons and the methylene peaks all meet downfield between 4.3 and 2.9ppm.
The methylene group of ethyl acetate accounting for two of the 6.61 protons is also included. There are three protons in this region resulting from ethanol – the OH and the methylene. Hence, ethanol correlates to 2.25 protons in the integral. The rest of the 2.36 protons can be attributed to water.
| Integrals |
Assignment |
Integral of 1 proton |
Mass (g) |
Volume (mL) |
% Composition (v/v) |
| 6.61 |
3.00 |
5.25 |
Total |
- |
- |
Total = 162.71 |
100 |
| 2.00 |
3.00 |
3.00 |
Ethyl acetate |
1.00 |
88.11 |
97.68 |
60.0 |
| 2.25 |
0 |
2.25 |
Ethanol |
0.75 |
34.55 |
43.79 |
26.9 |
| 2.36 |
0 |
0 |
Water |
1.18 |
21.24 |
21.24 |
13.1 |
Blue Nail Polish Remover
The blue nail polish remover’s spectrum is almost the same as that of the pink. However, it has another peak at 1.8 ppm. This peak alone is additionally seen in the spectrum. Acetone is the only reference solvent including just one peak. This implies that the blue nail polish remover has ethanol, ethyl acetate, acetone and water. The superimposition of the below spectra validates these findings.
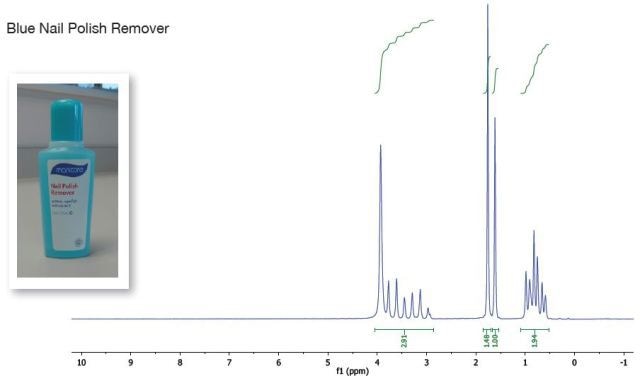
Figure 3. Blue nail polish remover spectrum
It can be seen that the rubbing alcohol has just one component. The spectrum is the same as that of isopropanol.
| Integrals |
Assignment |
Integral of 1 proton |
Mass (g) |
Volume (mL) |
% Composition (v/v) |
| 1.03 |
1.00 |
6.29 |
Total |
- |
- |
Total = 76.46 |
100 |
| 1.03 |
1.00 |
6.29 |
Isopropanol |
1 |
60.1 |
76.46 |
100 |
Vinegar’s NMR spectrum has two peaks. The 5ppm large peak may indicate water. The other peak is a singlet. Two reference solvents, having a separate, up-field singlet - acetone and acetic acid, are present. The acetone peak is anticipated to seem more shielded, about 1.5ppm. The peak present in the reference solvent of acetic acid is 1.3ppm. However, since acetic acid is organic, there may be a variation in the chemical shift with pH and concentration.
For acetone, identical behavior is not anticipated, hence, vinegar includes acetic acid and water. Vodka’s spectrum is the same as that of ethanol. However, since there is an integration of the exchangeable proton for lot more than 1, it may also include water. Methylated spirit spectrum is the same as that of ethanol.
Benefits of the Spinsolve™ benchtop NMR spectrometer
The Spinsolve™ benchtop NMR spectrometer is used for improving the teaching experience in a chemistry classroom or laboratory.
The benefits of the Spinsolve™ for educational requirements:
Cost Reduction
- In comparison to high field, low cost to purchase
- Possible to use non-deuterated solvents
- Low power consumption
- It is possible to use Budget NMR tubes
Time Saving
- Nearby – It easily fits on any laboratory bench
- Rapid sample exchange is possible with standard 5mm NMR tubes
- Fast – Students can work on it conveniently
- Easy to use – Innovative and simple software
- Safe – There is no stray magnetic field
Informative
- Allows guiding NMR education
- It is possible to acquire high-resolution NMR data in just 10s
- It is now available with 2D, multi-pulse experiments (2DJRes, COSY) and 19F Fluorine
- With NMR, teaching staff and students gain hands-on experience
This information has been sourced, reviewed and adapted from materials provided by Magritek.
For more information on this source, please visit Magritek.