Recycling of polymers is a growing industry, as many plastic containers and bottles can be recycled to form new products that traditionally would of end up in landfills.
Polymer Identification Techniques
Polymers tend to be mutually immiscible. This makes it difficult to clearly identify the types of polymers in a mixture. A polyethylene terephthalate (PET) can be easily mistaken as polycarbonate (PC). By using a Spectrum Two™ Fourier Transform Infrared (FT-IR) in conjunction with a Universal Attenuated Total Reflectance (UATR) accessory (Figure 1), chemical identification of the polymer can be performed easily.
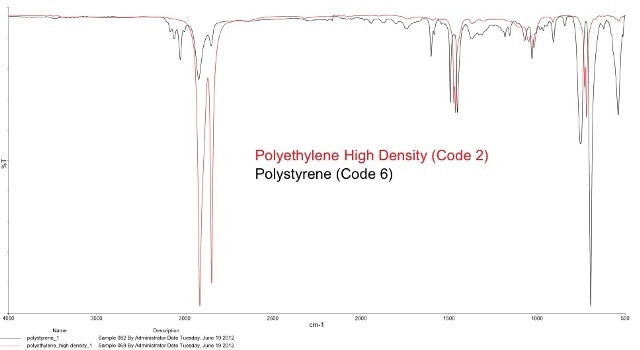
Figure 1. Overlay of PE and PS spectra in the FT-IR. FT-IR is a fast and accurate way to detect chemical differences in polymers.
However spectroscopic chemical identification alone will not be sufficient to establish the polymer sample classification. For instance, grades 2 and 4 are chemically the same polymer (polyethylene) as they exhibit similar FT-IR spectra. Figure 2 illustrates the similar spectra of grade 2 high density polyethylene (HDPE) and grade 4 low density polyethylene (LDPE).
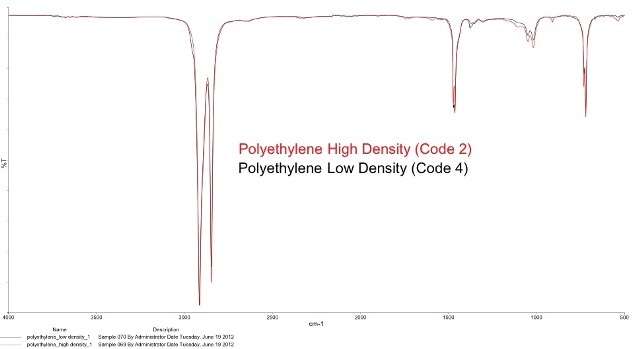
Figure 2. Overlaying Code 2 and 4, both polyethylenes, show the identical spectrum in the FT-IR. Other methods are needed to see the differences.
Measuring the low density and high density polyethylenes with differential scanning calorimetry (DSC) helps differentiate the polyolefin grades as illustrated in Figure 3. DSC can identify not only the correct form of polymer, but it can also determine when a finished product contains a physical mixture of polymers. An example of this can be seen with multilayer thin films.
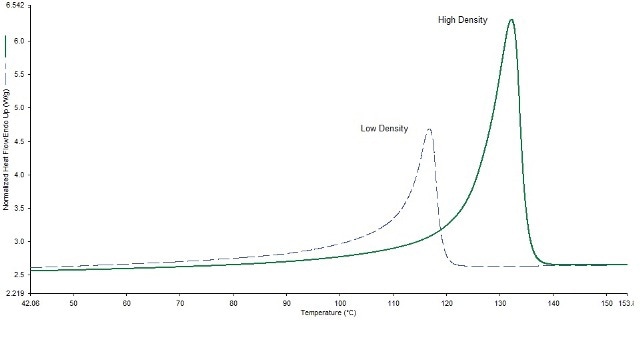
Figure 3. Grades 2 and 4 in the DSC show strong differences.
DSC measurement makes it easy to measure and establish the identity of the constituents, and in some cases, their concentrations (Figure 4). In this example, it was
determined that the weight percent of HDPE in the thin film was between 12-14%.
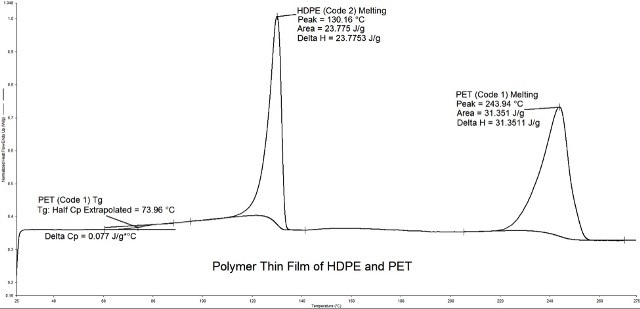
Figure 4. Thin film analysis by DSC, detecting the presence of two polymers within the sample.
With the aid of thermogravimetric analysis (TGA) , establishing the quantity of fillers e.g., talc, calcium carbonate, and glass fibers, used in materials, is no longer an issue.
The compendium library is provided with data files that describe the application method of TGA to establish the quantity of fillers in a polymer (Figure 5).
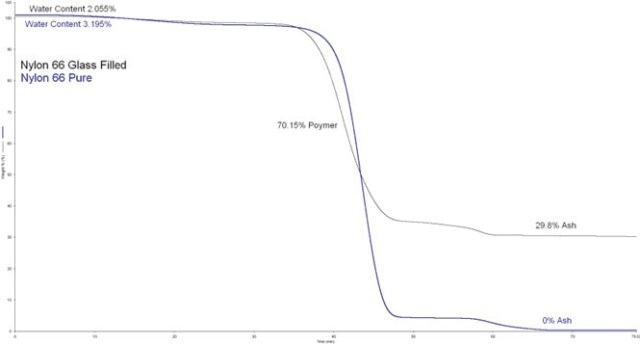
Figure 5. Detecting a filler in nylon
The Polymer Recycling Resource Pack
The Polymer Recycling Resource Pack comprises a polymer compendium that is capable of providing answers to how to spot the different types grades used in recycling and other basic issues one might come across during the process. There are three libraries of data files. Each library deals with one of the methods - FT-IR, TGA, and DSC.
Therefore using the compendium, one can easily identify the chemicals in the material, and derive very accurate data on the material quality or state. Furthermore, the compendium is very capable of providing solution to various issues and challenges relating to data interpretation associated with the recycling industry.

This information has been sourced, reviewed and adapted from materials provided by PerkinElmer.
For more information on this source, please visit PerkinElmer.