In the recent times, bisphenol A or BPA (Figure 1) has been gaining attention as studies report its negative effects on human health and safety. Initially, concerns regarding BPA focused only on baby bottles but now it includes all types of bottles.
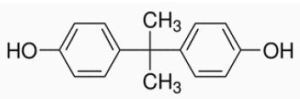
Figure 1. Structure of Bisphenol A (BPA)
BPA is used in the manufacture of common polymers such as polycarbonate and PVC. PVC is used extensively in products such as children’s toys (Figure 2), building materials and medical devices. BPA is used as a polymerization inhibitor during the manufacture of PVC. After polymerization is completed there is a chance residual BPA is still present.

Figure 2. Children's toy samples analyzed for BPA
Polycarbonate has many beneficial properties such as heat resistance and optical clarity. In the manufacture of polycarbonate, BPA acts as a monomer. However not all of the BPA is consumed and there is a chance of it leaching out of the polymer. Nowadays, polycarbonate is being substituted with new copolymers such as co-polyester to eliminate BPA.
Due to growing concerns about BPA on humans the levels of BPA in specific products, such as childrens toys and baby bottles, is being analyzed in specific products. To establish the existence and quantity of BPA in plastic materials, simple and rigorous test techniques are required. The extraction and HPLC analysis methods of children’s products for BPA are described in this article.
Experiment
The experiment involves BPA extraction from a toy matrix and subsequent UHPLC analysis. The extraction process involves simulation of the contact routes via which children may come into contact with BPA. For this experiment, two different extraction methods were selected to analyze the presence of BPA in 30g samples for each extraction.
In the first method, the sample was soaked in 1L of water for 24 hours at a temperature of 40°C (EN 14372). In the second method, the sample was soaked in 1 L HCl (0.07M) for 2 hours at a temperature of 37°C. Using a PerkinElmer Flexar™ FX-10 UHPLC system equipped with a PerkinElmer series 200a fluorescence detector, the extracted samples were tested. The separation process was achieved on a Brownlee validated C8 column (Table 1).
Table 1. HPLC Conditions for the analysis of BPA
| HPLC System |
PerkinElmer Flexar FX-10 UHPLC |
| Injection Volume |
50 µL |
| Column |
PerkinElmer C8 (150 mm x 4.6 mm, 5 µm) |
| Mobil Phase |
Methanol/Water (65/35) |
| Flow Rate |
1 mL/min |
| Detector Wavelength |
Excitation – 275 nm / Emission – 313 nm |
| Detector Response Time |
0.1 sec |
| PMT, Em BDW |
Super High, Wide |
| Run Time |
15 min |
Results
The BPA tested with the specified LC conditions eluted at 5.43mins (Figure 3). The UHPLC system was calibrated spanning a range of 1-50 ppb (µg/L) BPA (Table 2).
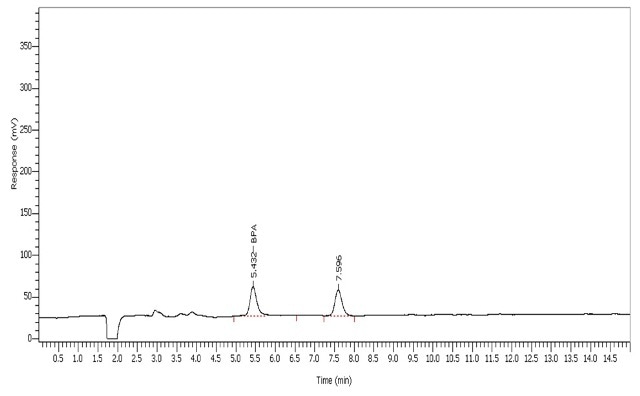
Figure 3. BPA calibration standard at 1 ppb
Table 2. Table for the analysis of BPA across the range of 1 - 50ppb (µg/L)
| Concentration |
Response |
| 1 ppb |
54163 |
| 10 ppb |
378051 |
| 20 ppb |
820335 |
| 40 ppb |
1548750 |
| 50 ppb |
1957851 |
| r2 |
0.9993 |
The BPA’s limit of quantitation (LOQ) with the method elaborated in this article is 1 ppb.
The signal-to-noise ratio at the LOQ is around 10:1. The response spanning the calibration range matched a linear calibration with an r2 value of 0.9993. Blanks, which were tested between standards and samples, revealed that the product did not contain any BPA contamination or residue. Toy sample extracts containing BPA were quantified using the calibration curve created during standard evaluation (Table 3). The chromatogram of the water extract of the toy dwarf sample is illustrated in Figure 4.
Table 3. Results from toy sample analysis
| Sample |
Extraction Type |
µg/L |
µg/g |
| Cube |
water |
2.04 |
0.068 |
| Cube |
HCl |
ND |
ND |
| Die |
water |
3.35 |
0.111 |
| Die |
HCl |
1.56 |
0.052 |
| Dwarf |
water |
4.32 |
0.144 |
| Dwarf |
HCl |
1.78 |
0.059 |
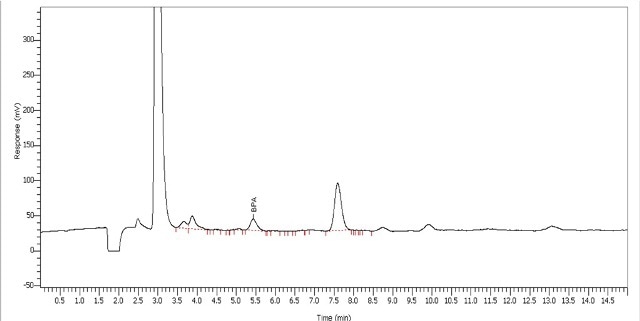
Figure 4. Analysis of toy dwarf for BPA using water
In the BPA extraction method, where the toy was heated in water for 24 hours at 40°C, a higher amount of BPA was extracted from the matrix than another BPA extraction method performed in acid. It was observed that BPA was present in all three water extraction processes in the calibration range of the standard curve.
Conclusion
As health concerns over exposure to BPA are raised, its analysis in plastics is becoming very important. The PerkinElmer Flexar FX-10 UHPLC system provides a sensitive and robust platform for this analysis. Demonstrated here was a calibration of BPA across a range of 1 – 50 ppb with a chromatographic run time of less than 10 minutes. This analysis was applied to 3 toy samples and BPA was identified in each sample.

This information has been sourced, reviewed and adapted from materials provided by PerkinElmer.
For more information on this source, please visit PerkinElmer.