Most materials are capable of crystallizing in various forms called polymorphs, which vary in the 3D arrangement of molecules or atoms in the crystal lattice. Polymorphs have similar chemical properties, but they are often characterized by different physical properties. In order to yield the right form, their crystallization should be performed in a controlled way. This process requires in situ monitoring of crystals.
Different types of polymorphs can be effectively and quickly differentiated using Raman spectroscopy. The disparity can be detected by the disappearance or appearance of typical Raman bands or a change in their given wavenumber positions. Based on application needs, Raman spectroscopy can be utilized with a non-contact probe or an in situ immersion probe. Moreover, Raman spectroscopy is non-destructive and does not require any sample preparation. This article shows how Raman spectroscopy is used to track the preferential crystallization of CaCO3 polymorphs in the existence of polymeric additives (Table 1).
Table 1. Polymeric additives used in this work.
| Polymeric Additive |
Abbreviation |
| Acusol® |
|
| Poly(aspartic acid) |
PAspAcid |
| Poly(acrylic acid) |
PAA |
| Polymaleimide synthesized by KOH initiator |
PMI_KOH |
| Polymaleimide synthesized by PbO and t-butyl benzyl alcohol initiator |
PMI_PbO |
| Polymaleimide synthesized by bis(triphenylphosphine) Cu(I) nitrate and t-butyl benzyl alcohol initiator |
PMI_Cu_sys |
Calcite (rhombohedral), vaterite (hexagonal), and aragonite (orthorhombic) are the three CaCO3 polymorphs with successive descending order of thermal stability.
Experimental Procedure
For the experiment, crystallizations were performed in a solution of Na2CO3 (500 mL, 100 0ppm with regard to carbonate) using polymeric additive (1.4 ppm) in an automated lab reactor at 25°C temperature. The reaction solution was added with CaCl2 solution (54 mL, 6250 ppm) at a speed of 1 mL/5 minutes, and the mixture was stirred by the reactor at 400 rpm. A HoloLab™ 5000 Raman analyzer equipped with a 785 nm Invictus™ laser was used to acquire Raman spectra at an interval of every 10 minutes. The results obtained from Raman spectroscopy were validated by x-ray diffraction (XRD) on grab samples.
Calibration
Peak intensity ratios were used to calibrate both XRD and Raman data. In respect to the Raman data, the peaks related to the carbonate plane bending modes of calcite (711 cm–1) and vaterite (690 cm–1) were utilized, as shown in Figure 1. Incidentally, aragonite is formed only at more than 90°C temperatures. Using known concentrations of vaterite and calcite, a calibration curve was created by ratioing peak intensities. The resulting data were accommodated to a second-order polynomial do as to acquire the calibration equation (Figure 2).
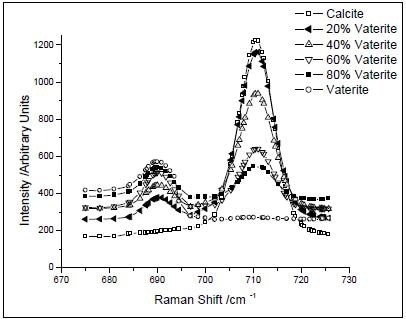
Figure 1. Raman spectra of prepared mixtures of CaCO3 polymorphs. The intensities of the bands at 690 and 711 cm–1 vary with the concentration of calcite and vaterite, respectively. (Reprinted with permission from Ref. 1. Copyright © 2003 American Chemical Society.)
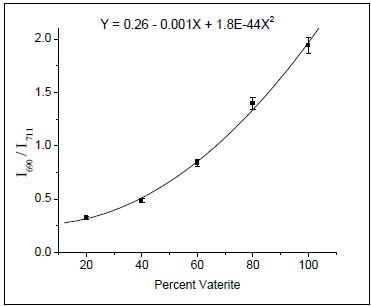
Figure 2. Calibration curve obtained by ratioing the 690 and 711 cm–1 Raman bands. (Reprinted with permission from Ref. 1. Copyright © 2003 American Chemical Society.)
Results
Figure 3 shows the results obtained from the experiments. The phase equilibrium does not seem to be affected by PMI_PbO, whereas the other two polymeric additives such as poly(acrylic acid) and Acusol®, led mostly to vaterite. In the experiment involving Acusol, it seems that calcite has not at all formed in the solution, whereas poly(acrylic acid) vaterite is selectively formed during crystallization. Vaterite is never formed with PMI_Cu_sys.
Other forms of polymeric additives provided results between these extremes, but only partly influencing the phase equilibrium.
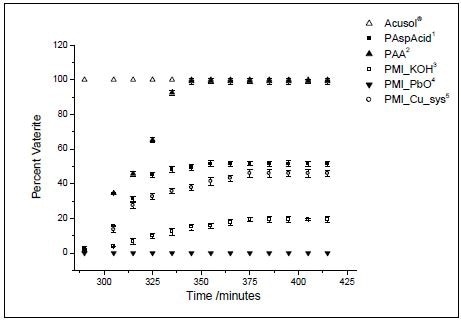
Figure 3. Percent vaterite during the course of the experiments, as determined by Raman spectroscopy. (Reprinted with permission from Ref. 1. Copyright © 2003 American Chemical Society.)
The unique axial transmissive spectrograph design of the analyzer makes it possible to acquire the entire spectrum at the same time, instead of successively scanning across portions of the spectral range. This was imperative because heterogeneous reaction mixture was used and this was being stirred quickly. Hence, scanning would lead to varied areas of a spectrum which is obtained from different materials as they travel through the probe’s focal cylinder.
Conclusion
Raman spectroscopy provides an easy and effective option to more conventional techniques for polymorph determination. Its utility can be attributed to the results determined by XRD and Raman spectroscopy, which agreed to less than 2%. This excellent agreement also demonstrates that quantification is not affected by particle size effects. Raman spectroscopy can be used as an online control technique for detecting polymorphs during the course of crystallization. It has the required chemical specificity to improve process understanding such as detecting novel intermediates or polymorphs during the monitoring of reactions, optimization, and scale-up steps of drug formulation.

This information has been sourced, reviewed and adapted from materials provided by Kaiser Optical Systems, Inc.
For more information on this source, please visit Kaiser Optical Systems, Inc.