Aflatoxins are the best known group of mycotoxins produced as secondary metabolites by fungi, primarily by Aspergillus parasiticus and Aspergillus flavus, and also to a smaller extent by other strains. This origin is also where the name Aspergillus flavus toxin is derived from.
It is possible to produce aflatoxins on crops in the field or during storage of agricultural products, particularly under high humidity and warm conditions. These substances can unfortunately persist for a long time, even after the fungi is killed, and thus contaminate foods.
Most mycotoxins are stable compounds that are not destroyed during cooking or food processing. Despite the huge quantities of aflatoxins, only a limited number are significant in (analytical) practice.
Aflatoxin B1 is extremely widespread and is present in food and feed products such as cottonseed, corn, and peanuts. It is highly toxic and has been classified as a group 1 carcinogen by the WHO. The aflatoxins B2, G1, and G2 are usually found accompanying B1, in lower concentrations in the contaminated samples. The order of toxicity is AFB1 > AFG1 > AFB2> AFG2.1
Ochratoxin A (OTA) is a mycotoxin formed from varied species of Aspergillus and Penicillium. OTA can be generated during plant growth, but it is often generated during food conversion and storage.4 Deoxynivalenol (DON) is one type of mycotoxin developed by specific Fusarium species that often infect rice, barley, oats, wheat, corn and various other grains during storage or in the field.
The exposure risk to humans is indirectly through food of animal origin (eggs, milk, liver, kidney) or directly through foods of plant origin (cereal grains). It has been found in triticale, sorghum, buckwheat, and other food products such as beer, malt, pancakes, popcorn, infant foods, noodles, breakfast cereals, bread, and flour.
DON affects human and animal health causing dizziness, headache, abdominal pain, diarrhea, vomiting, acute temporary nausea, and fever.3 Zearalenone (ZON) is a mycotoxin that belongs to the genus Fusarium, and it is produced mainly in food and feed. The development of effective analytical methods for identification and quantification is important as mycotoxins can easily enter the market and can pose a risk to public health. These methods are used on a large scale by governmental institutions and health protection agencies to monitor commercial animal feed and food products. In the good processing industry the same methods are used to check raw materials and products, in order to direct them to countries with suitable legislation.5
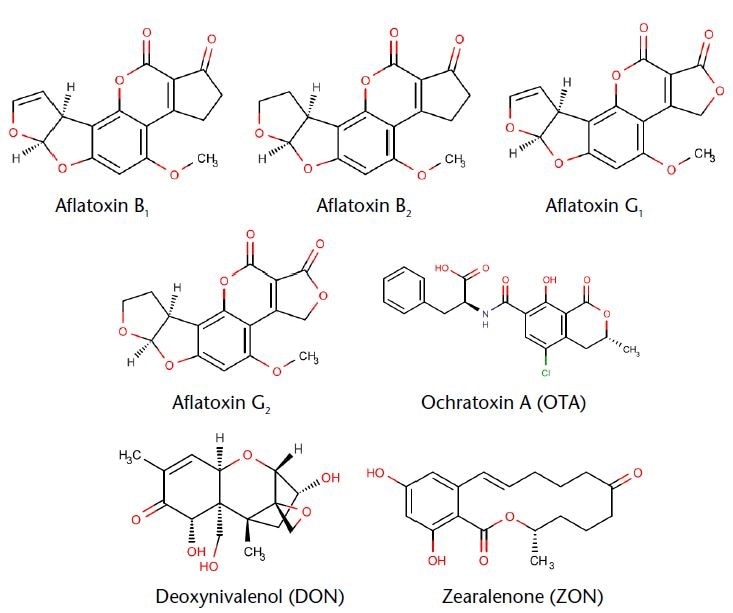
Figure 1. Chemical structure of the determined aflatoxins.
Countries throughout world control the presence of aflatoxins B1, B2, G1, and G2 in a wide range of unprocessed and processed foods. Chart 1 depicts exemplarily action limits set by the U.S. Food and Drug Administration (levels where the FDA will undertake legal action to remove products from the market). Charts 2 to 5 display limits for ZON, OTA, DON and maximum aflatoxin levels set by the European Commission according to regulation EG 1881/2006 5,6.
Chart 1. FDA action levels for aflatoxin in human food, animal feed and animal feed ingredients5
| Intended use |
Grain, grain by-product, feed or other products |
Aflatoxin level [µg/kg] |
| Human consumption |
Milk |
0.5 |
| Human consumption |
Foods, peanuts and peanut products, brazil and pistachio nuts |
20 |
| Immature animals |
Corn, peanut products and other animal feeds and ingredients, excluding cottonseed meal |
20 |
| Dairy animals, animals not listed above, or unknown use |
Corn, peanut products, cottonseed, and other animal feeds and ingredients |
20 |
| Breeding cattle, breeding swine and mature poultry |
Corn and peanut products |
100 |
| Finishing swine 100 pounds or greater in weight |
Corn and peanut products |
200 |
| Finishing (i.e., feedlot) beef cattle |
Corn and peanut products |
300 |
| Beef, cattle, swine or poultry, regardless of age or breeding status |
Cottonseed meal |
300 |
Chart 2. Maximum levels for DON in foodstuffs set by The European Commission6
| Foodstuff |
Maximum level [µg/kg] |
| Unprocessed cereals other than durum wheat, oats and maize |
1250 |
| Unprocessed durum wheat and oats |
1750 |
| Unprocessed maize |
1750 |
| Cereals intended for direct human consumption, cereal flour (including maize flour, maize meal and maize grits, bran as end product marketed for direct human consumption and germ |
750 |
| Pasta (dry) |
750 |
| Bread (including small bakery wares), pastries, biscuits, cereal snacks and breakfast cereals |
500 |
| Processed cereal-based foods and baby foods for infants and young children |
200 |
Chart 3. Maximum levels for certain contaminants in foodstuffs set by The European Commission6
| Foodstuff |
Maximum Aflatoxin Level [µg/kg] |
| B1 |
Sum of B1, B2, G1, G2 |
| Groundnuts to be subjected to sorting, or other physical treatment, before human consumption or use as an ingredient in foodstuffs |
8.0 |
15.0 |
| Nuts to be subjected to sorting, or other physical treatment, before human consumption or use as an ingredient in foodstuffs |
5.0 |
10.0 |
| Groundnuts and nuts and processed products thereof, intended for direct human consumption or use as an ingredient in foodstuffs |
2.0 |
4.0 |
| Dried fruit to be subjected to sorting, or other physical treatment, before human consumption or use as an ingredient in foodstuffs |
5.0 |
10.0 |
| Dried fruit and processed products thereof, intended for direct human consumption or use as an ingredient in foodstuffs |
2.0 |
4.0 |
| All cereals and all products derived from cereals, including processed cereal products |
2.0 |
4.0 |
| Maize to be subjected to sorting or other physical treatment before human consumption or use as an ingredient in foodstuffs |
5.0 |
10.0 |
| Raw milk, heat-treated milk and milk for the manufacture of milk-based products |
- |
- |
Following species of spices:
Capsicum spp. (dried fruits thereof, whole or ground, including chillies, chilli powder, cayenne and paprika) Piper spp. (fruits thereof, including white and black pepper), Myristica fragrans (nutmeg), Zingiber officinale (ginger), Curcuma longa (turmeric) |
5.0 |
10.0 |
| Processed cereal-based foods and baby foods for infants and young children |
0.10 |
- |
| Infant formulae and follow-on formulae, including infant milk and follow-on milk |
- |
- |
| Dietary foods for special medical purpose intended specifically for infants |
0.10 |
- |
Chart 4. Maximum levels for OTA in foodstuffs set by The European Commission6
| Foodstuff |
Maximum Level [µg/kg] |
| Unprocessed cereals |
5.0 |
| All products derived from unprocessed cereals, including processed cereal products and cereals intended for direct human consumption |
3.0 |
| Dried vine fruit (currants, raisins and sultanas) |
10.0 |
| Roasted coffee beans and ground roasted coffee, excluding soluble coffee |
5.0 |
| Soluble coffee (instant coffee) |
10.0 |
| Wine (including sparkling wine, excluding liqueur wine and wine with an alcoholic strength of not less than 15 % vol) and fruit wine |
2.0 |
| Aromatized wine, aromatized wine-based drinks and aromatized wine-product cocktails |
2.0 |
| Grape juice, concentrated grape juice as reconstituted, grape nectar, grape must and concentrated grape must as reconstituted, intended for direct human consumption |
2.0 |
| Processed cereal-based foods and baby foods for infants and young children |
0.50 |
| Dietary foods for special medical purposes intended specifically for infants |
0.50 |
| Green coffee, dried fruit other than dried vine fruit, beer, cocoa and cocoa products, liqueur wines, meat products, spices and liquorice |
- |
Chart 5. Maximum levels for ZON in foodstuffs set by The European Commission6
| Foodstuff |
Maximum level [µg/kg] |
| Unprocessed cereals other than maize |
100 |
| Unprocessed maize |
200 |
| Cereals intended for direct human consumption, cereal flour, bran as end product marketed for direct human consumption and germ |
75 |
| Maize intended for direct human consumption, maize flour, maize meal, maize grits, maize germ and refined maize oil |
200 |
| Bread (including small bakery wares), pastries, biscuits, cereal snacks and breakfast cereals, excluding maize snacks and maize based breakfast cereals |
50 |
| Maize snacks and maize based breakfast cereals |
50 |
| Processed cereal-based foods (excluding processed maize-based foods) and baby foods for infants and young children |
20 |
| Processed maize-based foods for infants and young children |
20 |
Theoretical: Recommended Sample Preparation
In addition to the sampling and grinding procedure, the traditional technique for aflatoxin analysis includes an extraction step, sample clean-up using solid-phase extraction (SPE) via high-performance liquid chromatography (HPLC). However, this technique limits sample throughput due to the time-consuming extraction and clean-up steps. Figure 2 gives an overview over the general steps employed in aflatoxin analysis.
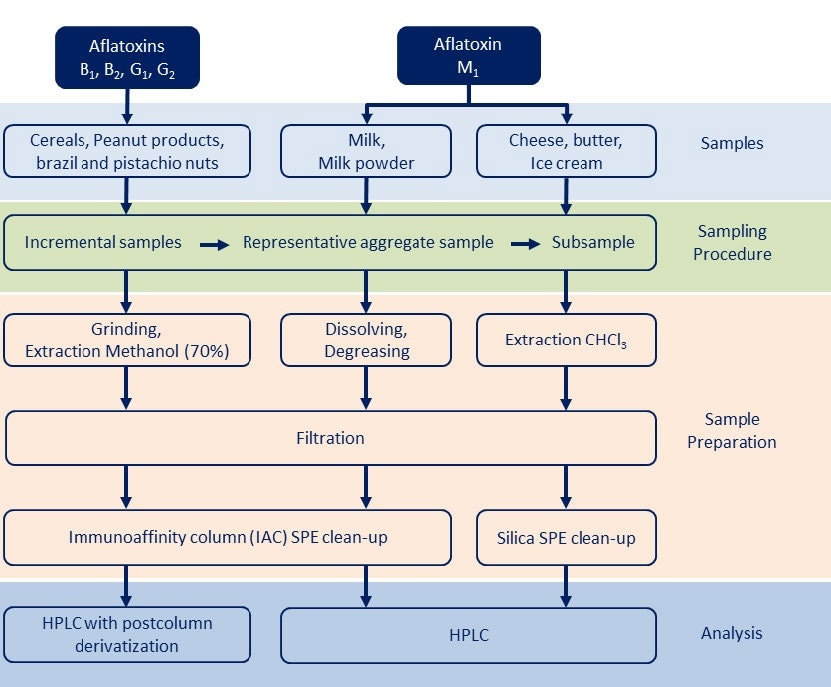
Figure 2. General analysis procedure for aflatoxin determination.
Method Parameters
Aflatoxins B1 and G1 are difficult to detect as they only show minimal fluorescence. Irradiating the aflatoxin mixture with UV light of 254 nm, the aflatoxins B1 and G1 go through photo-induced hydroxylation and are then measured through fluorescence spectrometry more sensitively.
| |
|
| Column |
Eurospher II 100-3 C18, 250 x 4 mm ID with precolumn |
| Eluent A |
Water |
| Eluent B |
Acetonitrile |
| Gradient |
Time [min] |
% A |
% B |
| 0.00 |
60 |
40 |
| 6.00 |
60 |
40 |
| 6.50 |
20 |
80 |
| 10.00 |
10 |
90 |
| 11.00 |
10 |
90 |
| Flow rate |
1.5 ml/min |
| Injection volume |
5 µl |
| Column temperature |
40°C |
| UV detection |
220 nm (20 Hz) |
| FLD detection |
Ex 329 nm Em 460 nm
(5 Hz, 0.1 s, Sensitivity: High, Gain 16)
Post column derivatization with UVE photochemical reactor at 100 µA |
Results
The FLD chromatogram with post column derivatization using the UVE reactor is shown in Figure 3. Figure 4 shows the DAD chromatogram for the same run where DON is detected. The separation works well on Eurospher II 100-3 C18 column. Peaks are baseline separated and also exhibit a good shape.
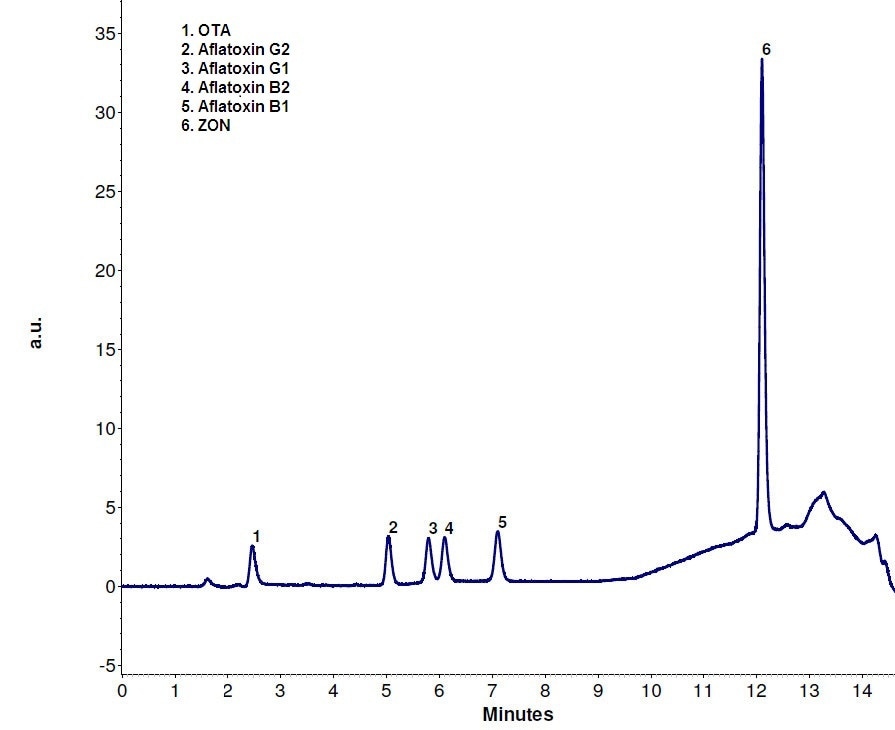
Figure 3. 1 FLD chromatogram for the mycotoxin analysis using UVE photochemical reactor for post column derivatization
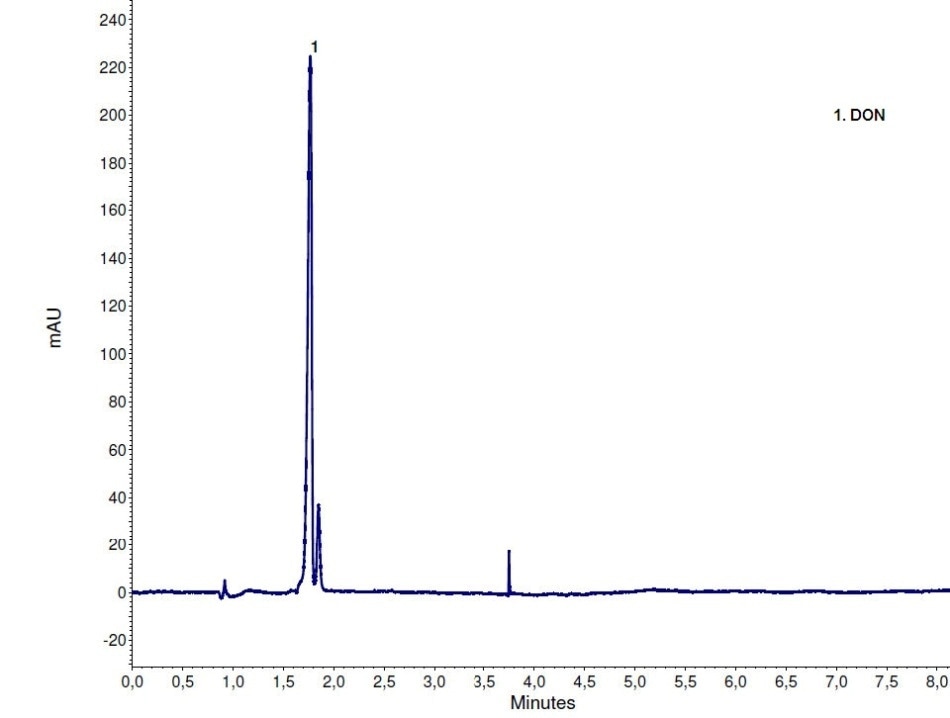
Figure 4. DAD chromatogram at 220 nm for the mycotoxin analysis using UVE photochemical reactor for post column derivatization
Conclusion
DON, ZON, and the 4 aflatoxins B1, B2, G1 and G2 were detected in a single chromatographic run using the UVE photochemical reactor for post column derivatization coupled with the Eurospher II C18 column and the AZURA Analytical HPLC system. It is necessary to further elucidate the determination of OTA.
The analysis of other substances is not affected as the mobile phase is not altered for this post column derivatization method. The photochemical reactor was handled easily as additional chemicals for derivatization were not needed. This application presents a simple alternative for the determination of mycotoxins.
See the White Paper for More Information
Physical Properties of the Recommended Column
| Stationary phase |
Eurospher II 100-3 C18 |
| USP code |
L 1 |
| Pore size |
100 Å |
| Pore volume |
0.8 ml/g |
| Specific surface |
320 ± 20 m2/g |
| Particle size |
3 µm |
| Form |
Spherical |
| % C |
16 |
| Endcapping |
Yes |
| Dimensions |
250 x 4 mm ID with precolumn |
| Order number |
25WE181E2G |

Recommended Instrumentation
| Description |
Order number |
| P 6.1L, binary HPG pump, 5 ml pump head |
APH35GA |
| DAD 6.1L |
ADC11 |
| CT 2.1 |
A05852 |
| AS 3950 |
A50070 |
| Fluorescence Detector RF-20 Axs |
A59201 |
| LCTech UVE photochemical reactor |
A07547 |
See the White Paper for More Information
References and Further Reading
- Derivatization reactions for the determination of aflatoxins by liquid chromatography with fluorescence detection, W.Th. Kok, Journal of Chromatography B, 659 (1994) 127-137 2
- http://www.eurofins.de
- Deoxynivalenol and its toxicity, Pavlina Sobrova,1 Vojtech Adam,1 Anna Vasatkova,2 Miroslava Beklova,3 Ladislav Zeman,2 and Rene Kizek1 1Department of Chemistry and Biochemistry, Faculty of Agronomy, Mendel University in Brno, Zemedelska 1, CZ- 613 00 Brno, Czech Republic 2Department of Animal Nutrition and Forage Production, Faculty of Agronomy, Mendel University in Brno, Zemedelska 1, CZ-613 00 Brno, Czech Republic 3Department of Veterinary Ecology and Environmental Protection, Faculty of Veterinary Hygiene and Ecology, University of Veterinary and Pharmaceutical Sciences, Palackeho 1-3, CZ-612 42 Brno, Czech Republic
- Review on the toxicity, occurrence, metabolism, detoxification, regulations and intake of zearalenone: an oestrogenic mycotoxin, Zinedine A1 , Soriano JM, Moltó JC, Mañes J., 1 Laboratory of Food Toxicology, National Institute of Health (INH), BP 769 Agdal, 27 Avenue Ibn Battouta, Rabat, Morocco
- FDA Mycotoxin Regulatory Guidance, National Grain and Feed Association 1250 Eye St., N.W., Suite 1003, Washington, D.C., 20005-3922 August 2011
- COMMISSION REGULATION (EC) No 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs, Official Journal of the European Union, L 364/5 - L 364/24, 20.12.2006

This information has been sourced, reviewed and adapted from materials provided by KNAUER Wissenschaftliche Geräte GmbH.
For more information on this source, please visit KNAUER Wissenschaftliche Geräte GmbH.