This article focuses on studying the surface acidic properties of oxide catalysts and carriers (γ -Al2O3, CeO2, ZrO2, SiO2, TiO2, HZSM5 zeolite), comparatively probing their surfaces by ammonia temperature-programmed desorption (ATPD) measurements. ATPD is a reliable and simple technique in which a surface, following saturation with ammonia at low temperature, is subject to a temperature ramp, which leads to desorption of the probe molecule together with a temperature profile.
By quantitatively and/or qualitatively analyzing the desorption pattern, it is possible to attain information about the desorption/adsorption energy and the quantity of ammonia that has been adsorbed on the surface (ammonia uptake). As a basic molecule, ammonia can be used as a probe to examine surface acidity. This data can help comprehend the catalytic behavior of a sample, or even help in fine tuning the synthesis of new systems. Instead of using a traditional TCD Detector for this task, a Quadrupole Mass Spectrometer (Hiden HPR-20 QIC) was used by connecting it through a heated capillary to the testing apparatus.

The Hiden HPR-20 QIC in the Lab used for characterization and testing of catalytic samples.
Ammonia Temperature Programmed Desorption
The use of a QMS allowed us to easily discriminate between the different species desorbing from our surfaces, without the use of any kind of chemical or physical filter and traps which could negatively affect the analyses. Properly tuning the ionization potential of the instrument helped prevent water molecule fragmentation and related interference with the ammonia m/z signal. The accuracy and reliability of ammonia temperature-programmed desorption data was analyzed by theoretical criteria and experimental tests highlighting the effects of data acquisition mode, carrier gas, particle size and reactor geometry, remarking the flexibility of the technique used.
All the studied materials featured complex ATPD patterns spanned in the range 423-873K, except for ceria showing a resolved and narrow desorption peak, indicative of homogeneous weak acidity. Quantitative data signal a difference of more than one order of magnitude in ammonia uptake between the other materials and silica. Since the ATPD profiles of ceria match Gaussian curves irrespective of surface coverage and heating rate, the patterns of the studied materials are described as linear combinations of four Gaussian functions related to medium, weak, strong and extremely strong site populations. After collecting all the profiles, ATPD modeling analyses were applied that helped obtain information about the energy of adsorption of the probe molecule related to each desorbing temperature. The cumulative energy site distributions indicated the following acidity scale based on the average energy (expressed in kJ/mol) value (e.g., surface coverage θ = 0.5)
CeO2 (100) < γ -Al2O3 (111) ≈ ZrO2 (111) ≈ TiO2 (117) ≈ SiO2 (118) < HZSM5 (135)
Conclusion
Dehydration of isopropanol was performed on propylene as a probe reaction to obtain additional information on the functionality of the studied materials. The results obtained matched the ones earlier uncovered by ATPD measurements in terms of strength and abundance of surface acid sites, but also made it possible to distinguish between Brønsted and Lewis acidic sites.
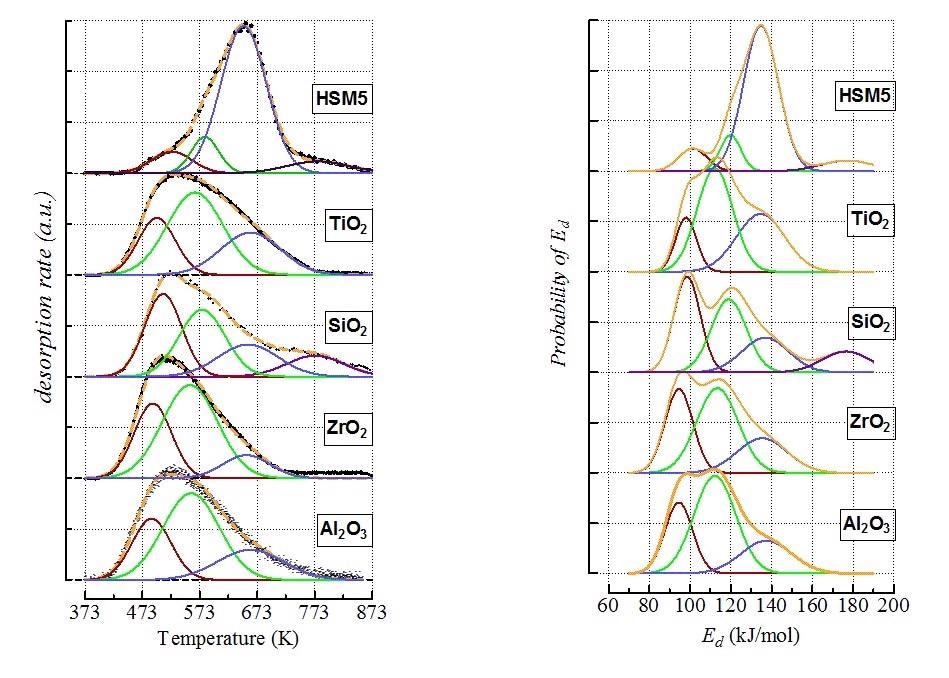
Figure 1. (Left) Deconvolution of ATPD profiles by Gaussian functions (dotted yellow lines depict the generated profiles, while black points are the experimental data); (Right) Energy distribution functions of ammonia desorption from the various site populations.
Project Summary By:

Roberto Di Chio
Dipartimento di Ingegneria,
Università degli Studi di Messina,
Contrada Di Dio, Sant'Agata,
I-98166 Messina,
Italy
Paper Reference
Francesco Arena, Roberto Di Chio, Giuseppe Trunfio (2015) “An experimental assessment of the ammonia temperature programmed desorption method for probing the surface acidic properties of heterogeneous catalysts” Applied Catalysis A: General 503, 227-236

This information has been sourced, reviewed and adapted from materials provided by Hiden Analytical.
For more information on this source, please visit Hiden Analytical.