
Organic waste, through biomass conversion, can be used for the synthesis of various chemicals and for energy production. One of the synthesis methods is through biogas to syngas catalytic conversion, by CO2 reforming of CH4. For the methane reforming reaction, all metals of the VIIIA group on a large variety of supports have been examined. Particularly, when nickel is well dispersed on the support, it exhibits good catalytic activity
Experiment
Catalyst Preparation
12 ml water solution containing 0.153 g Nickel acetate tetrahydrate was used to impregnate1.0 g silica support (SBA-15). Post sonication for several minutes, the mixture was stirred at room temperature for 1 hour. The silica that was impregnated was then collected and dried at 100 ˚C in vacuum and calcined at 550 ˚C for 2 hours with heating rate of 10 ˚C min-1. This process was repeated multiple times to increase the Ni content. At the end of 2 cycles, the Ni content was approximately 5%, whereas after 5 cycles, it was about 10-11%.
Characterization
Temperature programed reaction (TPRx CH4:CO2 = 1.5:1), oxidation (O2-TPO), and reduction (CH4-TPR) experiments were carried out using a CATLAB microreactor – MS system (Hiden Analytical, UK) on fresh and in situ reduced Ni catalysts. Heating rates were 10 ˚C min-1. In situ reduction was carried out under a H2 flow by heating to 550 ˚C at 10 ˚C min-1 and maintaining at this temperature for 1 hour. XRD data were acquired on a Bruker D8 diffractometer.
Results and Discussion
Figures 1 and 3 depict the TPR results of the 5 and 11% Ni samples, respectively to have high activity with CH4 reduction taking place at approximately 580 ˚C in both samples. The 11% Ni sample displays significantly more consumption of CH4 as would be predicted due to the higher Ni content.
The TPRx results of pre-reduced 5 and 11% Ni samples are shown in Figures 2 and 4. In the 5% Ni sample, conversion of CH4 + CO2 → 2H2 + 2CO happens at around 450 ˚C with total conversion of the CO2 taking place near 700 ˚C.
In the 11% Ni sample, conversion of CH4 + CO2 → 2H2 + 2CO takes place at approximately 400 ˚C with total conversion of the CO2 happening at a similar temperature of the 5% sample at around 700 ˚C
The results indicate that as Ni content increases, the temperature at which reaction starts decreases, although complete conversion of the CO2 occurs at a comparable temperature.
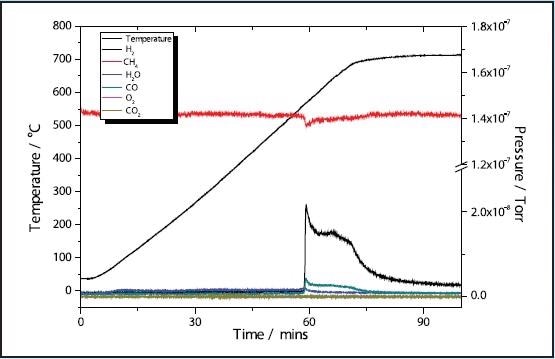
Figure 1. 5% Ni CH4 TPR.
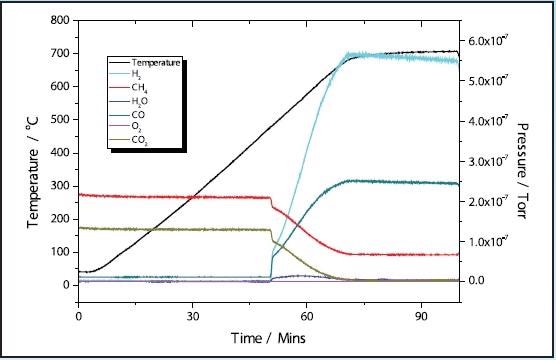
Figure 2. 5% Ni CH4 TPRx.
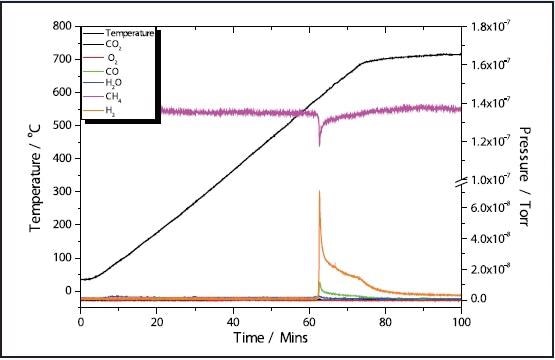
Figure 3. 11% Ni CH4 TPR.
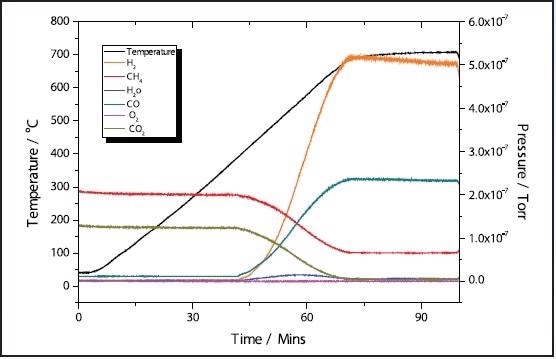
Figure 4. 11% Ni CH4 TPRx.
Figure 5 shows the low-angle XRD results, which demonstrate the existence of well-ordered mesopores having long-range order and retention of the mesostructure following introduction of nickel species. Before and after impregnation, mesoporous nickel-containing silica and the mesoporous silica all have similar structure as reported [1-2]. The major drawback of the post-synthetic method is that the loadings of Ni can frequently cause the deterioration of the mesostructure or the blockage of mesochannels by large guest Ni (NiO) particles. In this experiment, the mesostructures experienced only slight shrinkages upon loading of nickel species, as denoted in Figure 5, which might be effected by the thermal treatment after each nickel loading. The retention of the higher angle peaks evidently signifies that the mesostructure did not deteriorate considerably. This can be credited to the thicker, more robust framework walls of SBA-15, which provide good thermal and hydrothermal stability during the preparation.
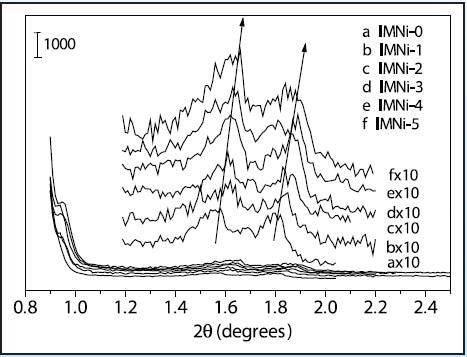
Figure 5. Low-angle XRD patterns of nickel containing mesoporous silicas after each impregnation cycle (a) 0 cycles, (b) 1 cycle, (c) 2 cycles, (d) 3 cycles, (e) 4 cycles, (f) 5 cycles.
Conclusions
- TPReaction demonstrates the high activity and selectivity of these catalysts
- Methane activation occurred at relatively low temperatures
- Ni is well dispersed inside the mesopores
- Textural and structural properties of the mesoporous support are retained independent of the Ni loading
References
- (a) D. Zhao, J. Feng, Q. Huo, N. Melosh, G. H. Fredrickson, B. F. Chmelka and G. D. Stucky, Science 1998, 279, 548.
(b) D. Zhao, Q. Huo, J. Feng, B. F. Chmelka and G. D. Stucky, J. Am. Chem. Soc. 1998, 120, 6024.
- (a) W. Wang, M. Song, Micro. Meso. Mater. 2006, 96, 255.
(b) W. Wang, M. Song, J. Non-Crystal. Solids, 352, 2006, 3153.
(c) W. Wang, M. Song, Mater. Res. Bull., 41(2), 2006, 436.

This information has been sourced, reviewed and adapted from materials provided by Hiden Analytical.
For more information on this source, please visit Hiden Analytical.