Through biomass conversion, organic waste can be used for the synthesis of a variety of chemicals and for energy production. Biogas to syngas catalytic conversion, by CO2 reforming of CH4, is one of the synthesis routes. All metals of the VIIIA group on a wide variety of supports have been studied for the methane reforming reaction. Ni demonstrates good catalytic activity, particularly when well dispersed on the support. It was observed that plasma modification of Ni samples increased the dispersion of these catalysts [1].
A microreactor based on the Hiden CATLAB was used to perform plasma modification of a Ni/Al2O3/CeO sample. The reactor was designed to allow a catalyst to be heated under controllable gas flow and temperature conditions. Figure 1 shows a schematic illustration of the reactor. Besides the standard furnace arrangement, a dielectric barrier discharge (DBD) could also be generated over the length of the catalyst.

Figure 1. Plasma reactor.
The DBD consisted of an outer cylindrical metallic electrode wrapped around a quartz tube and connected to ground, and an inner coaxial tungsten wire electrode of 1.0 mm diameter. The tungsten electrode is connected to the open-circuit end of the secondary winding of a high-voltage transformer operated at 50 kHz. While the plasma was running, the catalyst was loaded into the reactor and flushed with He for 1.5 hours. Subsequently, the sample was transferred to a standard CATLAB reactor for characterization using TPR and reaction testing.

Results and Discussion
The results of a TPR experiment on both the plasma modified and unmodified Ni samples are shown in Figures 2 and 3. While the two TPR are similar, the plasma modified sample seems to have a broader main reduction peak. Further study is necessary to establish any additional differences.
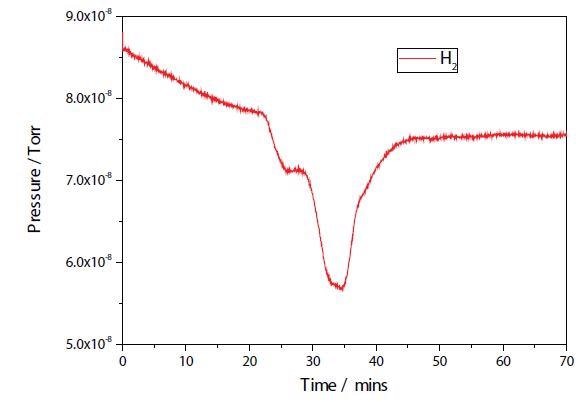
Figure 2. Ni/Al2O3/CeO TPR.
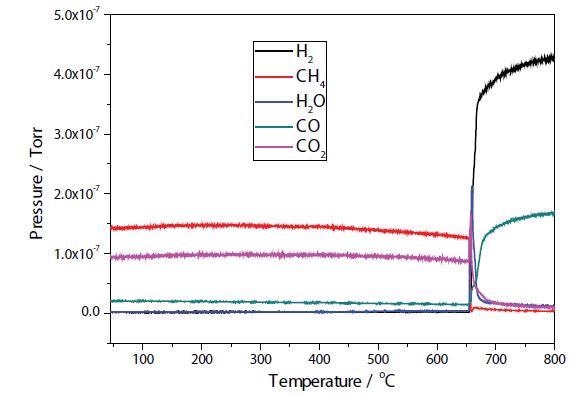
Figure 3. Ni/Al2O3/CeO TPRx.
Figures 4 and 5 depict the TPRx results of plasma modified and unmodified Ni samples. In the unmodified sample, conversion of CH4 + CO2 → 2H2 + 2CO takes place at approximately 650 °C, with total conversion of the CO2 occurring at around 700 °C. Conversion of CH4 + CO2 → 2H2 + 2CO occurs at around 600 °C in the modified Ni sample.
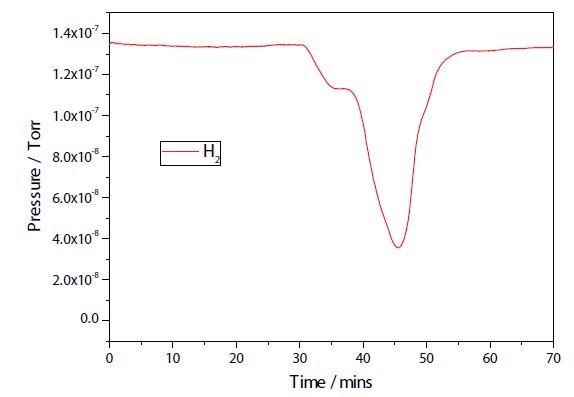
Figure 4. Plasma modified Ni/Al2O3/CeO TPR.
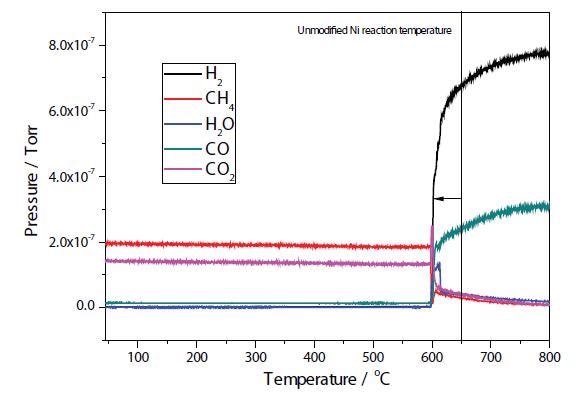
Figure 5. Plasma modified Ni/Al2O3/CeO TPRx.
Conclusions
- Plasma modification demonstrates the potential for preferentially altering the reactive properties of the catalyst.
- TPReaction shows the high activity and selectivity of these catalysts.
- Methane activation occurred in plasma modified and unmodified Ni samples.
References
- Y. Zhu, Z, Li, Y, Zhou, H. Wang, Journal of Natural Gas Chemistry 14 (2005) 1–3.

This information has been sourced, reviewed and adapted from materials provided by Hiden Analytical.
For more information on this source, please visit Hiden Analytical.